Research News
Jul 11, 2024
- Medicine
Phage-Derived Enzyme Targets E. faecalis Biofilms to Mitigate Acute Graft-Versus-Host Disease
Researchers from Japan discover a new enzyme with promising antibacterial activity
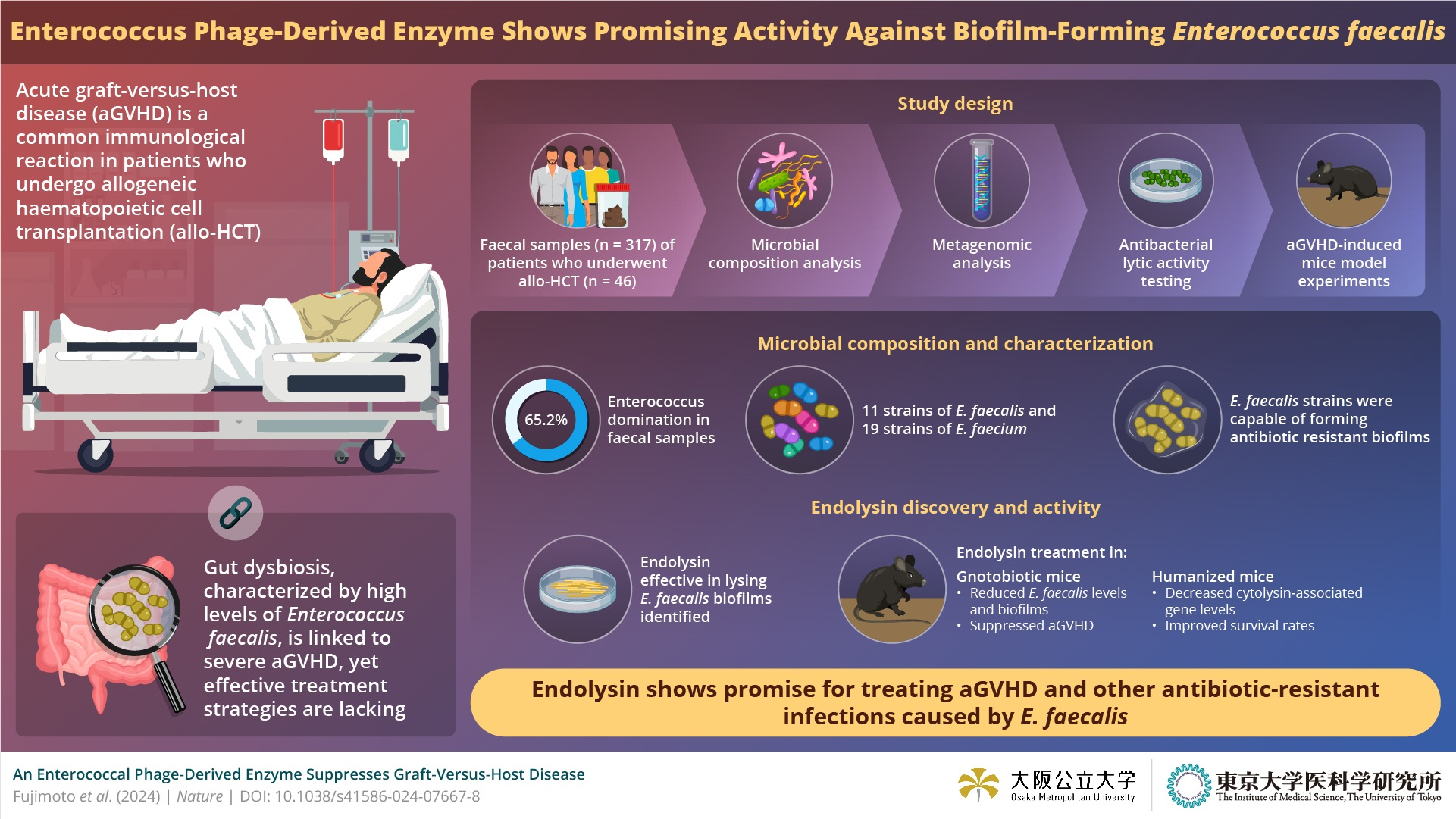
Endolysin, a phage-derived antibacterial enzyme demonstrated specific activity against pathogenic Enterococcus bacteria.
Identification of bacteriophage-derived enzyme and its antibacterial activity can contribute to novel therapies that target biofilm-forming bacteria and help in the treatment against acute graft-versus- host disease.
Credit: Kosuke Fujimoto, Seiya Imoto, and Satoshi Uematsu from The University of Tokyo
Allogenic haematopoietic cell transplantation (allo-HCT) involves transferring healthy donor stem cells to recipients with conditions like blood cancer, bone marrow failure, or certain genetic blood disorders. Acute graft-versus-host disease (aGVHD) is a common complication, where the donor's immune cells attack the recipient's tissues. Recent studies highlight the significant role of the microbiome in aGVHD, with dysbiosis contributing to its pathogenesis. Dysbiosis can lead to the emergence of pathogenic commensal bacteria including Enterococcus species, particularly E. faecalis and E. faecium, which are associated with multidrug-resistant infections in allo-HCT patients. However, there is a lack of effective therapies specifically tailored to treat dysbiosis in the context of aGVHD.
To address this gap, a multidisciplinary team led by Associate Professor Kosuke Fujimoto from Osaka Metropolitan University and The University of Tokyo, alongside Professor Seiya Imoto from The University of Tokyo, and Satoshi Uematsu from Osaka Metropolitan University and The University of Tokyo, conducted an in-depth analysis of the intestinal bacteriome of allo-HCT patients. The study aimed to investigate the prevalence and implications of Enterococcus domination in this specific patient population. Their findings, published on July 10, 2024, in the journal Nature, shed light on crucial aspects of gut microbiota dynamics in the context of allo-HCT.
Explaining the motivation behind the present research, Fujimoto says, “During dysbiosis, some symbiotic commensal bacteria acquire pathogenic characteristics, proliferate, and become directly involved in the onset and progression of the disease. Recognizing the specificity of phage therapy and its ability to spare beneficial bacteria from adverse effects, we focused our research on phage-derived lytic enzymes.”
The team initiated their investigation by examining the intestinal microbiome of allo-HCT patients, where they noted a predominance of Enterococcus species, particularly E. faecalis. This was notably associated with acute leukemia. Despite being sensitive to several antibiotics, E. faecalis strains possessed cytolysin-associated genes, indicating high virulence. Further exploration through metagenomic analysis revealed the presence of genetic signatures associated with biofilm formation. They then proceeded with whole-genome sequencing of E. faecalis. This unveiled the presence of an intriguing bacteriophage-derived enzyme known as endolysin, exhibiting potent antibacterial activity specifically targeting E. faecalis.
Fujimoto and his team conducted rigorous in-vitro and in-vivo assays to confirm the efficacy of the endolysin. They found that it exhibited narrow-spectrum activity against E. faecalis and effectively lysed biofilms. Notably, the endolysin's lytic activity did not affect other intestinal bacteria species. In mouse models, the efficacy of the endolysin was assessed in two experiments. Firstly, mice with induced aGVHD were treated with the endolysin, resulting in a significant reduction of E. faecalis colonization in faeces and suppression of aGVHD development. In the second experiment, mice with a gut microbiota resembling that of humans, dominated by Enterococcus bacteria, were treated with the endolysin, leading to decreased Enterococcus levels and improved survival rates.
“Bacteriophage research is gaining momentum, with advancements in phage therapy paving the way for new treatments. Our discovery of the endolysin enzyme holds promise for future applications in preventing or treating acute GVHD,” says Fujimoto, expressing his optimism regarding the potential impact and real-life applications of the research work.
Thanks to the research team for the identification of endolysin from bacteriophage, a new class of therapeutic compounds targeting highly resistant, biofilm-forming bacteria is now possible!
This article has been summarized in Nature’s Research Briefings on Oct. 3, 2024:
https://www.nature.com/articles/d41586-024-03188-6
Video
Funding
This study was supported by the Japan Agency for Medical Research and Development (AMED) (to K.F., JP21ae0121048; and to S.U., JP21fk0108619 and JP21ae0121040), the Japanese Society for the Promotion of Science (JSPS) (to K.F., 22K16329; to S.U., 21K19495 and 22H00477), the Takeda Science Foundation (to S.U.), the Canon Foundation (to S.U.), and the Center of Innovation Program from the Japan Science and Technology Agency (JST) (to S.U. and S.I., JPMJCE1304).
Paper Information
Journal: Nature
Title: An enterococcal phage-derived enzyme suppresses graft-versus-host disease
DOI: 10.1038/s41586-024-07667-8
Author: Kosuke Fujimoto, Tetsuya Hayashi, Mako Yamamoto, Noriaki Sato, Masaki Shimohigoshi, Daichi Miyaoka, Chieko Yokota, Miki Watanabe, Yuki Hisaki, Yukari Kamei, Yuki Yokoyama, Takato Yabuno, Asao Hirose, Mika Nakamae, Hirohisa Nakamae, Miho Uematsu, Shintaro Sato, Kiyoshi Yamaguchi, Yoichi Furukawa, Yukihiro Akeda, Masayuki Hino, Seiya Imoto, and Satoshi Uematsu
Published: 10 July, 2024
https://www.nature.com/articles/s41586-024-07667-8
https://doi.org/10.1038/s41586-024-07667-8
Contact
Graduate School of Medicine
E-mail: kfujimoto[at]omu.ac.jp
*Please change [at] to @.
SDGs
