Research Results
Jul 27, 2021
- Press Release
- Joint Research Paper
A press release announcing the joint research findings of Assoc. prof. Masazumi Tamura (OCU), Tohoku University and Japan Steel Corporation has been issued
The world's first successful direct synthesis of plastic from atmospheric carbon dioxide has been achieved.
This research presentation was introduced in the following media.
- July 28, 2021 Japan Metal Daily, Nikkan Sangyo Shimbun, TeleTou BIZ
- July 29, 2021 The Nikkan Kogyo Shimbun
- July 30, 2021 @DIME
- August 4, 2021 The Chemical Daily
- September 7, 2021 Nikkei Business Daily
- September 25, 2021 Monthly Scientific Journals "Newton" November issue
- November 23, 2021 The Mainichi Newspapers
Summary
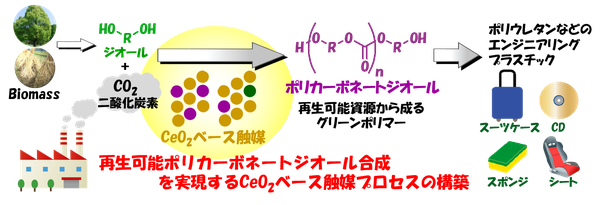
Associate Professor Masazumi Tamura (Research Center for Artificial Photosynthesis, Osaka City University), Professor Keiichi Tomishige (Department of Applied Chemistry, Graduate School of Engineering, Tohoku University), and Kenji Nakao (Section Chief, Advanced Technology Research Laboratory, Nippon Steel Corporation) have successfully developed a groundbreaking catalytic process for the direct synthesis of aliphatic polycarbonate diol from carbon dioxide under ambient pressure using a diol*1, without the need for a dehydrating agent. This achievement represents a world first. By incorporating a cerium oxide catalyst, the researchers demonstrated the ability to produce aliphatic polycarbonate diol with high yield and selectivity. Their findings have been published in the scientific journal "Green Chemistry".
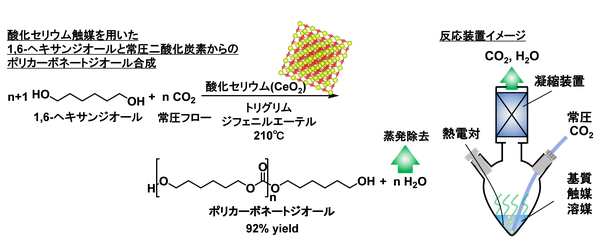
Polycarbonate diol is a crucial intermediate for the synthesis of polyurethanes, which are widely used in plastics. Currently, it is synthesized using toxic phosgene*2 or carbon monoxide as raw materials. However, there is a growing demand for alternative technologies that can replace these hazardous substances from a green chemistry*3 perspective. A method that uses carbon dioxide as an alternative raw material and reacts it with a diol to synthesize polycarbonate diol has attracted attention as a green reaction system that produces only water as a byproduct. However, achieving high yields has required the use of high-pressure carbon dioxide or dehydrating agents. The method discovered in this research overcomes these challenges. By using a cerium oxide catalyst and bubbling carbon dioxide at normal pressure into a diol, the generated water can be removed from the reaction system, enabling the highly selective and high-yield production of the desired polycarbonate diol.
This research has been published online in Green Chemistry (Impact Factor = 10.18) on Monday, July 26, 2021.
Comments from the researchers
 Addressing the global challenge of carbon dioxide reduction, this study presents a groundbreaking solid catalyst process for the direct conversion of carbon dioxide into polymers under ambient conditions. Our findings demonstrate that a cerium oxide catalyst can facilitate the reaction between carbon dioxide and a diol, yielding valuable polycarbonate diol without requiring dehydrating agents. Future efforts will focus on optimizing the catalyst and process for industrial implementation.
Addressing the global challenge of carbon dioxide reduction, this study presents a groundbreaking solid catalyst process for the direct conversion of carbon dioxide into polymers under ambient conditions. Our findings demonstrate that a cerium oxide catalyst can facilitate the reaction between carbon dioxide and a diol, yielding valuable polycarbonate diol without requiring dehydrating agents. Future efforts will focus on optimizing the catalyst and process for industrial implementation.
1. Background
Given the escalating impacts of climate change and natural disasters, the need to reduce carbon dioxide emissions, a primary greenhouse gas, has become a global imperative. International agreements such as the Paris Agreement have set ambitious targets and outlined plans to achieve these goals. Recognizing the potential of carbon dioxide as a C1 chemical feedstock*4, researchers have been actively exploring methods to convert it into valuable chemicals. This approach offers a promising avenue for carbon dioxide fixation.
Despite being a major greenhouse gas, carbon dioxide is a thermodynamically stable molecule, making its activation challenging. The development of highly efficient catalysts and optimized reaction conditions is crucial for effective carbon dioxide conversion. Non-reductive methods, which involve reactions that do not alter the oxidation state of the carbon atom in carbon dioxide, have emerged as promising strategies. By reacting carbon dioxide with alcohols or diols, valuable organic carbonates*5 and aliphatic polycarbonates can be produced. However, the conventional synthesis of these compounds relies on toxic reagents such as phosgene and carbon monoxide, necessitating the development of greener alternatives. For the long-term sequestration of carbon dioxide, its conversion into polymers is a particularly attractive approach.
A major challenge in the direct synthesis of polycarbonates from carbon dioxide and diols is the reaction's low yield (less than 1%) and the formation of significant amounts of water as a byproduct. While a catalytic system employing a nitrile as an organic dehydrating agent and cerium oxide has been reported, this approach suffers from several drawbacks. The requirement of high-pressure carbon dioxide, coupled with the difficulties associated with recovering and regenerating the dehydrating agent, limits its practical application. Additionally, side reactions involving the nitrile dehydrating agent can compromise product purity. These limitations underscore the need for a more sustainable and efficient catalytic process that does not rely on the use of dehydrating agents.
2. Research Topics
In the synthesis of polycarbonate diol from carbon dioxide and diol, water is produced as a byproduct, and its removal is essential for improving the yield. As a water removal method without using a dehydrating agent, we focused on the boiling point difference between the product, diol, and water and hypothesized that the desired carbonate synthesis would proceed by blowing in carbon dioxide at normal pressure to evaporate water. As a result, it was revealed that only cerium oxide exhibited high activity among various metal oxide catalysts, and we successfully developed a very simple catalytic reaction system that does not require a dehydrating agent or high-pressure carbon dioxide.
3. Expected effects
This technology provides a novel catalytic process that enables the chemical conversion of carbon dioxide under normal pressure without the use of additives. Furthermore, we believe that this technology can be applied to substrates with boiling points significantly higher than that of water, and can be expanded to the synthesis of organic carbonates, carbamates*6, and urea, which are useful as additives for lithium-ion batteries and raw materials for polymer synthesis. By establishing various chemical synthesis routes from carbon dioxide, this catalytic process is expected to contribute to the chemical fixation of carbon dioxide.
4. Future Developments
We will continue our research and development efforts, focusing on improving the solid catalyst for practical applications and scaling up the process.
5. Research Projects
This research was made possible through funding from the New Energy and Industrial Technology Development Organization (NEDO) under their "NEDO Leading Research Program / Untapped Challenge 2050".
Osaka City University and Tohoku University have been conducting ongoing research to develop solid catalysts for the conversion of carbon dioxide. Our previous work has highlighted the effectiveness of cerium oxide-based metal oxide catalysts for carbon dioxide activation. In this project, with the aim of utilizing atmospheric carbon dioxide from industrial sources such as blast furnaces, we have successfully developed a solid catalyst system that can directly synthesize polymers from carbon dioxide, eliminating the need for energy-intensive dehydrating agents.
Nippon Steel Corporation has set an ambitious goal of achieving carbon neutrality by 2050, as outlined in its “Nippon Steel Carbon Neutral Vision 2050: Challenging Zero Carbon Steel”. Our research offers a promising solution to this challenge by demonstrating the feasibility of converting carbon dioxide emissions from steel mills into valuable products. By developing a process that can capture and utilize carbon dioxide efficiently, our research contributes significantly to carbon dioxide fixation and reduction.
Terminology
- *1: Diol
- A general term for compounds in which two hydroxyl groups (OH groups) are attached to two different carbons
- *2: Phosgene
- Also known as carbonyl chloride, it is highly reactive and used as various raw materials. On other hand, it is also extremely toxic, causing severe irritation to the eyes and exhibiting asphyxiant toxicity to the human body.
- *3: Green Chemistry
- A concept and its associated technologies aimed at reducing the burden on human health and the environment throughout the design, synthesis, application, and disposal of chemical compounds and products.
- *4: C1 chemical raw materials
- Chemical raw materials that contain a single carbon atom, such as carbon dioxide, carbon monoxide, and methane.
- *5: Carbonate
- Compounds with the structure -O-CO-O- are commonly referred to as carbonate compounds or carbonic esters.
- *6: Carbamate
- A general term for carbamic acid ester compounds with the structure of >N-CO-O-. It is also called carbamate.
Publication Information
| Publications: | Green Chemistry (IF=10.18) |
|---|---|
| Title: | Direct synthesis of polycarbonate diols from atmospheric flow CO2 and diols without using dehydrating agents |
| Author: | Yu Gu, Masazumi Tamura,* Yoshinao Nakagawa, Kenji Nakao, Kimihito Suzuki, and Keiichi Tomishige* |
| URL: | https://doi.org/10.1039/d1gc01172c |