Research Results
Mar 17, 2020
- Press Release
- Joint Research Paper
A press release announcing the joint research findings of Prof. Tomoko Yoshida's group and Sakai Chemical Industry Co., Ltd. has been issued.
Development of a Safe and High-Yield Solution Synthesis Method for Black Phosphorus, a Next-Generation Material
– Accelerating the Dream Technology of Artificial Photosynthesis –
Summary
A research group led by Tomoko Yoshida (Professor, Deputy Director, Center for Artificial Photosynthesis, Osaka City University) , Akiyo Ozawa (Osaka City University) and Sakai Chemical Industry Co., Ltd. have collaboratively developed a highly efficient and simple solution-based method for synthesizing black phosphorus, which acts as a catalyst for generating hydrogen from water using solar energy. Black phosphorus, an allotrope of phosphorus, has attracted significant attention as a material capable of absorbing a large portion of the visible light spectrum. However, it has faced the challenge of difficult mass production, which is essential for industrial applications.
By employing a solution-based method, we have achieved high-yield synthesis of black phosphorus starting from safe and non-toxic red phosphorus, paving the way for mass production.
This article was published on the online page of the Journal of Materials Chemistry A, a journal of the Royal Society of Chemistry (RSC) on March 12, 2020 (Japan time).
Research Background
The pressing issue of global warming has spurred interest in hydrogen as a clean energy alternative to fossil fuels. Extensive research has been devoted to developing photocatalytic systems that can harness solar energy to split water molecules, producing hydrogen. Black phosphorus, a highly promising photocatalyst capable of harnessing a broad spectrum of sunlight from ultraviolet to near-infrared, has traditionally been synthesized using energy-intensive methods such as high-temperature and high-pressure techniques or chemical vapor deposition. These methods pose significant challenges for large-scale, low-cost production.
Solution-based synthesis has been identified as a potential pathway for the scalable and cost-effective production of black phosphorus. Recent research has reported the successful synthesis of black phosphorus from white phosphorus via solvothermal processes*1, which employ high-temperature or high-pressure solvents to facilitate the formation of solid-phase products. However, since white phosphorus is highly toxic, there was a need to develop a method to obtain black phosphorus in high yield from safe and non-toxic red phosphorus.
*1: A method for synthesizing materials through solvent heating reactions.
Outline of Research
We have developed a novel solvothermal method for the high-yield synthesis of black phosphorus from red phosphorus using ethylenediamine*2 as a solvent. Through comprehensive spectroscopic analysis, we have elucidated the reaction mechanism. Red phosphorus dissolves in ethylenediamine as trivalent phosphorus species, which then aggregate to form zero-valent polyphosphorus. The subsequent stacking of these polyphosphorus species within the solution leads to the formation of black phosphorus.
The synthesized sample showed a remarkably high content of black phosphorus, with a yield significantly enhanced from the conventional approximately 10% to around 90%. Moreover, when loaded with a co-catalyst*3, the sample exhibited excellent hydrogen production activity under visible light irradiation in a methanol aqueous solution, demonstrating its promise as a photocatalyst for water splitting. (Patent pending.)
*2: An organic compound that is miscible with both water and alcohol, and is widely used in chemical synthesis.
*3: To immobilize metal nanoparticles on a support for catalytic applications.
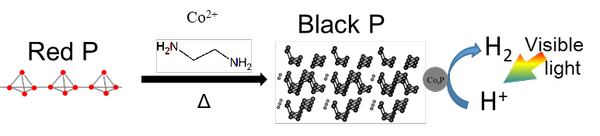
Expected effect
Black phosphorus, a layered material like graphite, is a promising 2D material for its ability to absorb light from visible to near-infrared wavelengths depending on layer thickness. Yet, its industrial use has been limited due to synthesis challenges. This study, by achieving high-yield synthesis of black phosphorus from safe red phosphorus, is expected to spur research on black phosphorus-based photocatalysts.
Furthermore, monolayer black phosphorus, or phosphorene, is a related material to graphene, the subject of the 2010 Nobel Prize in Physics. It exhibits excellent conductivity and, unlike graphene*4, possesses a bandgap, making it a semiconducting material.
*4: A thin-film polymer with a thickness of a single carbon atom. It has a honeycomb lattice structure composed of carbon atoms and their bonds.
Future Developments
Black phosphorus is plagued by the challenges of synthetic difficulty and poor air stability. Leveraging the synthesis guidelines for black phosphorus derived from this study, we intend to develop approaches for expanding the layer area of black phosphorus and for improving its stability.
Joint research, funding, etc.
This research is a collaborative study between Osaka City University and Sakai Chemical Industry Co., Ltd. It was conducted with the support of the funding listed below.
Grants-in-Aid for Scientific Research, New Academic Area Research 'Creation of Complex Anion Compounds and New Functions,' Research Project 16H06440.
Publication Information
| Publications: | Journal of Materials Chemistry A |
|---|---|
| Title of Paper: | Black phosphorus synthesized by solvothermal reaction from red phosphorus and its catalytic activity for water splitting |
| Author: | Akiyo Ozawa (Osaka City University, Sakai Chemical Industry Co., Ltd.), Muneaki Yamamoto (Osaka City University), Tetsuo Tanabe (Osaka City University), Saburo Hosokawa (Kyoto University), Tomoko Yoshida (Osaka City University) |
| URL: | https://doi.org/10.1039/C9TA13441G |