-
Flow Friedel-Crafts alkylation of 1-adamantanol with arenes using HO-SAS as an immobilized acid catalyst
Kasakado, T.; Hyodo, M.; Furuta, A.; Kamardine, A.; Ryu, I.; Fukuyama, T.;
J. Chin. Chem. Soc. 2020, 67, 2253-2257.
DOI: 10.1002/jccs.202000518
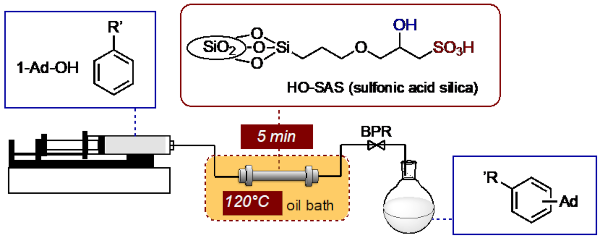
In this communication flow Friedel–Crafts alkylation was studied using hydroxy-substituted sulfonic acid-functionalized silica as a catalyst and 1-adamantanol as a model
substrate. The reaction of 1-adamantanol (1a) with toluene (2a) proceeded well with 5 min of residence time at 120°C to give good yield of 1-tolyladamantane (3a) as a 1:9
mixture of meta and para isomers. When the flow synthesis was carried out over 2.5 hr of running time, the collected five fractions contain the product 3a in 97–92% yields,
suggesting the durability of the catalyst.
-
Synthesis of Aliphatic Amides through a Photoredox Catalyzed Radical Carbonylation Involving Organosilicates as Alkyl Radical Precursors
Cartier, A.; Levernier, E.; Dhimane, A-L.; Fukuyama, T.; Ollivier, C.; Ryu, I.
Adv. Synth. Catal. 2020, 362, 2254 –2259.
DOI: 10.1002/adsc.202000314
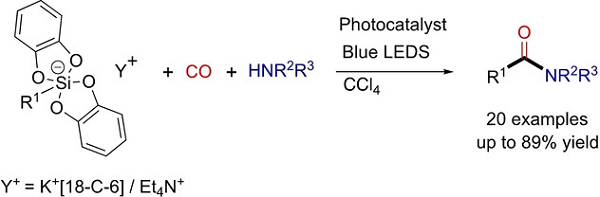
Alkyl radicals, from primary to tertiary, formed by photocatalyzed oxidation of organosilicates, are involved efficiently in radical carbonylation with carbon monoxide (CO), in the
presence of various amines and CCl4, leading to a variety of amides in moderate to good yields.
-
Pd/light-induced alkyl-alkenyl coupling reaction between unactivated alkyl iodides and alkenylboronic acids
Huang, H-J.; Wang, Y-T.; Wu, Y-K.; Ryu, I.
Org. Chem. Front. 2020, 7, 1266-1270.
DOI: 10.1039/d0qo00318b
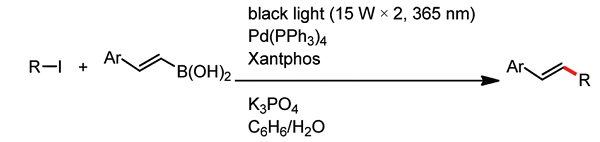
Alkyl–alkenyl coupling reaction between unactivated alkyl iodides and 2-arylalkenylboronic acids utilizing a Pd/light combined system was studied. This reaction provided 2-
alkylarylalkenes in good yields. It is proposed that in this reaction alkyl radicals are formed by SET from a Pd catalyst to alkyl iodides, which then undergo addition reactions to
form C–C double bonds of alkenyl boronic acids and the subsequent β-fragmentation of boronyl radicals leads to the formation of alkyl-substituted arylalkenes.
-
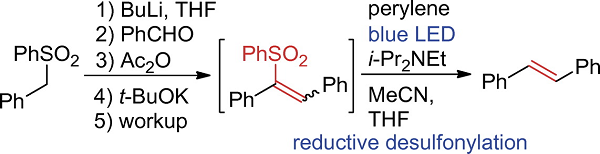
Syntheses of Diarylethenes by Perylene-catalyzed Photodesulfonylation from Ethenyl Sulfones
Watanabe, H.; Takemoto, M.; Adachi, K.; Okuda, Y.; Dakegata, A.; Fukuyama, T.; Ryu, I.; Wakamatsu, K.; Orita, A.
Chem. Lett. 2020, 49, 409-412.
DOI: 10.1246/cl.200046
Diarylethenes were obtained from the corresponding ethenyl sulfones by photocatalyzed desulfonylation using UV or blue LEDs. When perylene and i-Pr2NEt were used as a
photocatalyst and a sacrificing reagent, respectively, this desulfonylation proceeded smoothly to afford the desired ethenes with the functional groups such as chloro, alkoxy and
heteroaromatic rings remaining untouched. The use of a flow photoreactor enabled this desulfonylation to proceed more rapidly to finish in an hour of residence time.
-
Site-Selective C(sp3)–H Functionalization of Fluorinated Alkanes Driven by Polar Effects Using a Tungstate Photocatalyst
Fukuyama, T.; Nishikawa, T.; Ryu, I.
Eur. J. Org. Chem. 2020, 1424-1428.
DOI: 10.1002/ejoc.201901135.
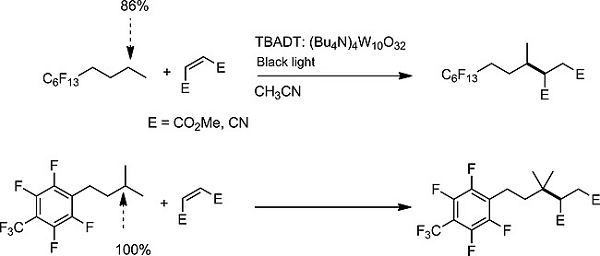
The TBADT-catalyzed C(sp3)–H functionalization of perfluorophenyl- and perfluoroalkyl-substituted alkanes was studied to determine how the fluorous substituents affect site-
selectivity. Alkylation of alkyl-substituted perfluorobenzene avoids α-C–H bonds, unlike their alkylbenzene counterparts, allowing site-selective functionalization of C–H bonds
remote to the aromatic ring. Alkylation of alkanes having a perfluoroalkyl group also avoided α-C–H bonds. Radical polar effects in the SH2 transition states could explain this
avoidance of α-C–H functionalization.