-
Using High-Power UV-LED to Accelerate a Decatungstate-Anion-Catalyzed Reaction: A Model Study for the Quick Oxidation of Benzyl Alcohol to Benzoic Acid Using Molecular
Oxygen.
Hyodo, M.; Iwano, H.; Kasakado. T.; Fukuyama, T.; Ryu, I.
Micromachines. 2021, 12, 1307-1315.
DOI: 10.3390/mi12111307
High-power UV-LED irradiation (365 nm) effectively accelerated the decatungstate-anion-catalyzed oxidation of benzyl alcohol 1 to benzoic acid 3 via benzaldehyde 2. As the
power of the UV-LED light increased, both the selectivity and yield of benzoic acid also increased. The reaction was finished within 1 h to give 3 in a 93% yield using 2 mol% of
decatungstate anion catalyst. The combination of a flow photoreactor and high-power irradiation accelerated the oxidation reaction to an interval of only a few minutes.
-
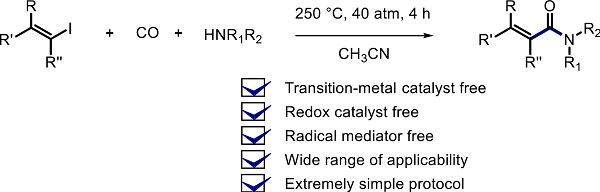
Electron-Catalyzed Aminocarbonylation: Synthesis of α,β-Unsaturated Amides from Alkenyl Iodides, CO, and Amines
Picard, B.; Fukuyama, T.; Bando, T.; Hyodo, M.; Ryu, I.
Org. Lett. 2021, 23, 9505-9509.
DOI: 10.1021/acs.orglett.1c03714
Aminocarbonylation of alkenyl iodides with CO and amines proceeded under heating to produce α,β-unsaturated amides in good yields (23 examples, 71% average yield). This
catalyst-free method exhibited good functional-group tolerance, and open a straightforward access to functionalized acrylamides, as illustrated by the synthesis of Ilepcimide. A
hybrid radical/ionic mechanism involving chain electron transfer is proposed for this transformation.
-
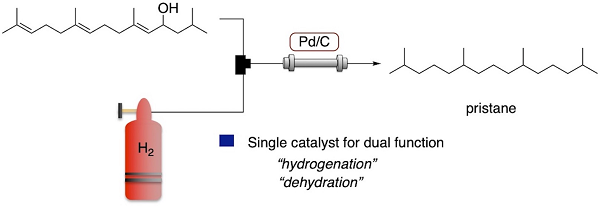
Greener Synthesis of Pristane by Flow Dehydrative Hydrogenation of Allylic Alcohol Using a Packed-Bed Reactor Charged by Pd/C as a Single Catalyst
Kasakado, T.; Hirobe, Y.; Furuta, A.; Hyodo, M.; Fukuyama, T.; Ryu, I.
Molecules 2021, 26, 5845.
DOI: 10.3390/molecules26195845
Our previous work established a continuous-flow synthesis of pristane, which is a saturated branched alkane obtained from a Basking Shark. The dehydration of an allylic
alcohol that is the key to a tetraene was carried out using a packed-bed reactor charged by an acid–silica catalyst (HO-SAS) and flow hydrogenation using molecular hydrogen via
a Pd/C catalyst followed. The present work relies on the additional propensity of Pd/C to serve as an acid catalyst, which allows us to perform a flow synthesis of pristane from
the aforementioned key allylic alcohol in the presence of molecular hydrogen using Pd/C as a single catalyst, which is applied to both dehydration and hydrogenation. The present
one-column-two-reaction-flow system could eliminate the use of an acid catalyst such as HO-SAS and lead to a significant simplification of the production process.
-
Blacklight-Induced Hydroxylation of Arylboronic Acids Leading to Hydroxyarenes Using Molecular Oxygen and Tetrabutylammonium Borohydride
Kawamoto, T.; Ryu, I.
Helv. Chim. Acta 2021, 104, e2100102.
DOI: 10.1002/hlca.202100102
A new simple protocol for the conversion of arylboronic acids to hydroxyarenes was achieved using molecular oxygen in the presence of tetrabutylammonium borohydride
under blacklight irradiation (360 nm). A radical chain mechanism in which a superoxide ion (O2−.) plays a key role is proposed.
-
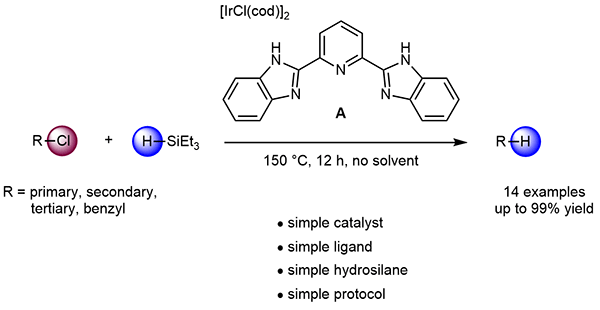
A New Protocol for Catalytic Reduction of Alkyl Chlorides Using an Iridium/Bis(benzimidazol-2'-yl)pyridine Catalyst and Triethylsilane
Fukuyama, T.; Hamada, Y.; Ryu, I.
Synthesis 2021, 53, 3404-3408.
DOI: 10.1055/a-1527-4526.
The reduction of alkyl chlorides using triethylsilane is investigated. Primary, secondary, tertiary, and benzylic C–Cl bonds are effectively converted into C–H bonds using an
[IrCl(cod)]2/2,6-bis(benzimidazol-2′-yl)pyridine catalyst system. This catalyst system is quite simple since the tridentate N-ligand can be easily prepared in one step from
commercially available reagents.
-
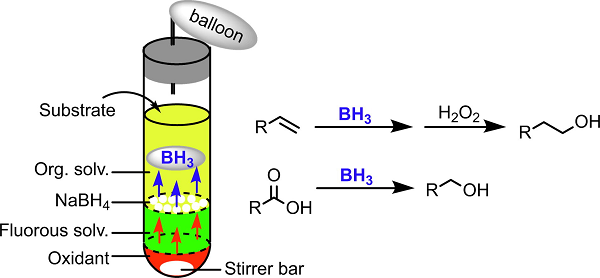
Borane evolution and its application to organic synthesis using the phase-vanishing method
Soga, N.; Yoshiki, T.; Sato, A.; Kawamoto. T.; Ryu, I.; Matsubara, H.
Tetrahedron Lett. 2021, 69.
DOI: 10.1016/j.tetlet.2021.152977
Although borane is a useful reagent, it is difficult to handle. In this study, borane was generated in situ from NaBH4 or nBu4NBH4 with several oxidants using a phase-vanishing
(PV) method. The borane generated was directly reacted with alkenes, affording the desired alcohols in good yields after oxidation with H2O2 under basic conditions. The
selective reduction of carboxylic acids with the evolved borane was examined. The organoboranes generated by the PV method successfully underwent Suzuki–Miyaura coupling.
Using this PV system, reactions with borane can be carried out easily and safely in a common test tube.
-
Site-Selective Alkenylation of Unactivated C(sp )-H Bonds Mediated by Compact Sulfate Radical
Ueda,M.; Kamikawa, K.; Fukuyama, T.; Wang. Y.-T.; Wu, Y.-K.; Ryu, I.
Angew. Chem. Int. Ed. 2021, 60, 3545–3550.
DOI: 10.1002/anie.202011992
A broad variety of unactivated acyclic and alicyclic substrates cleanly undergo site-selective alkenylation of unactivated C(sp3)−H bonds with 1,2-bis(phenylsulfonyl)ethene in
the presence of persulfate. This simple transformation furnishes (E)-2-alkylvinylphenylsulfones in up to 88 % yield. In contrast with the previously reported decatungstate
protocol, the current method is applicable to alkenylation of sterically hindered C−H bonds. This important advantage significantly broadens the substrate scope, and is attributed
to the compact size of the sulfate radical employed in the C−H activation and cleavage.





