論文 ( PUBLICATIONS )
2025 年 ( 令和 7 年 )
ABSTRACT: The epoxide-opening cascade biogenesis was originally proposed for ionophoric polyether antibiotics as in the Cane-Celmer-Westley hypothesis and has been utilized as an efficient method to rapidly construct natural polyether skeletons. For example, teurilene, the marine polyether derived from red algae, was synthesized from squalene tetraepoxide, a hypothetical biogenetic precursor, via a three-step, 5-exo selective epoxide-opening cascade cyclization triggered by Brønsted-acid-catalyzed hydrolysis of the terminal epoxide. In previous work, it was shown that the epoxide-opening cascade cyclization that produces tetrahydrofuran (THF) products in acidic aqueous media instead yields tetrahydropyran (THP) in neutral water. The THP formation proceeded via an epoxonium-ion intermediate by simple heating in neutral water. In this report, we successfully established a “ring-size-divergent” synthetic method to divergently construct five-, six-, and seven-membered ether rings from identical diepoxides, achieving the synthesis of seven-membered (oxepane) ring under acidic conditions using Lewis acid. With this new synthetic strategy, we also accomplished the divergent synthesis of nerolidol-type sesquiterpenoids and feroniellins, revising the proposed stereochemistry of certain natural products and determining their absolute configurations. Additionally, we evaluated the anti-inflammatory activities of the synthetic samples.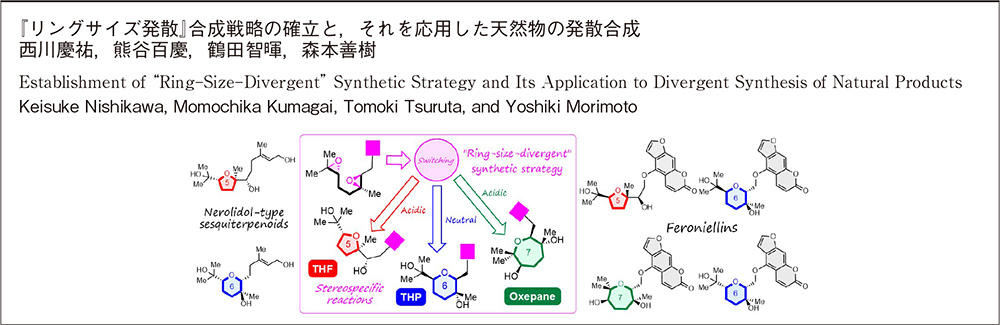
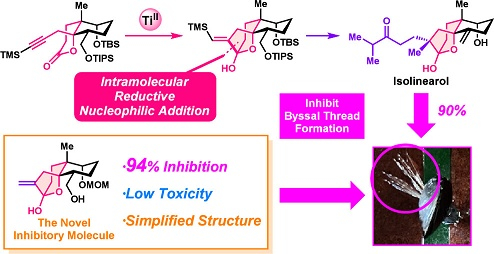
"Asymmetric Total Synthesis of Isolinearol Using Low-Valnece Titanium and Evaluation of Its Inhibitory Activity against Mussel Byssal Thread Formation" T. Tsuruta, K. Nishikawa, Y. Yoshino, D. Osada, T. Miwa, K.Nimura, T. Kamada, and Y. Morimoto Org. Lett. 27, 3489–3494 (2025).
Selected as Front Cover.
Highlighted in Synfacts 21, 336 (2025)
ABSTRACT: The asymmetric total synthesis of isolinearol, a seco-dolastane-type diterpenoid that inhibits byssal thread formation by mussels, has been achieved. In the synthesis, the key features include an intramolecular reductive nucleophilic addition using a low-valence titanium species and the direct installation of a ketone side chain. We evaluated their biological activities using the synthetic samples and found the novel inhibitory molecules with a simplified structure exhibit high inhibitory activities against byssus formation and low toxicities.

ABSTRACT: In this study, we explored anti-inflammatory compounds from the brown alga Dictyopteris polypodioides and isolated 7 meroterpenoids. Their anti-inflammatory activities were evaluated using the lipopolysaccharide-stimulated mouse macrophage cell line, RAW264. Yahazunol (1) exhibited similar nitric oxide (NO) production inhibitory activity as zonarol (2), which has previously been shown to be an anti-inflammatory compound. Yahazunol (1), zonarol (2), and isozonarol (3) inhibited not only NO production but also inducible nitric oxide synthase, interleukin-6, and C-C motif chemokine ligand 2 mRNA expression in RAW264 cells. The structure-activity relationships of the 11 compounds, including their synthetic analogs, revealed the significance of the hydroquinone moiety in the anti-inflammatory activity of these sesquiterpenoids in RAW264 cells. Diacetylated zonarol (9) exhibited an activity comparable to that of zonarol as a result of intracellular deacetylation. These results provide new insights into the anti-inflammatory activity of hydroquinone-containing natural products.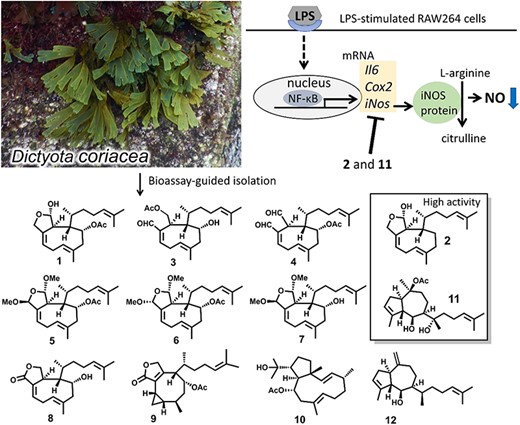
2024 年 ( 令和 6 年 )
ABSTRACT: The thyrsiferol family natural products are marine triterpene polyethers biogenetically derived from squalene and structurally characterized by a bromine atom and some six- and five-membered ethereal rings. Their stereostructures cannot easily be determined by modern spectroscopic analysis, because there are acyclic tetrasubstituted chiral centers and the remote stereoclusters. In these cases, asymmetric total synthesis demonstrates its power. Herein, to determine the entire stereostructure of the thyrsiferol family member isodehydrothyrsiferol, isolated from the red alga Laurencia viridis, the asymmetric total synthesis has been performed. The key steps are the convergent and effective synthetic strategy using a Suzuki–Miyaura cross-coupling, a one-pot construction of the tetrahydropyranyl C ring via a stoichiometric Katsuki-Sharpless asymmetric epoxidation and 6-exo oxacyclization in situ promoted by Ti chelation, and 6-endo bromoetherification for the A ring formation. Through the enantioselective total synthesis, we have accomplished complete assignment of the entire stereostructure for isodehydrothyrsiferol and found the absolute configuration of the ABC ring system is opposite to that common to the other congeners from the same red algae. In addition, such enantiodivergency also occurred between dehydrothyrsiferol and isodehydrothyrsiferol originating from the identical red alga Laurencia viridis. There are no these findings without asymmetric total synthesis.
ABSTRACT: In this study, we explored anti-inflammatory compounds from the brown alga Dictyopteris polypodioides and isolated 7 meroterpenoids. Their anti-inflammatory activities were evaluated using the lipopolysaccharide (LPS)-stimulated mouse macrophage cell line, RAW264. Yahazunol (1) exhibited similar nitric oxide (NO) production inhibitory activity as zonarol (2), which has previously been shown to be an anti-inflammatory compound. Yahazunol (1), zonarol (2), and isozonarol (3) inhibited not only nitric oxide production but also inducible nitric oxide synthase (iNOS), interleukin-6 (IL6), and C-C motif chemokine ligand 2 (CCL2) mRNA expression in RAW264 cells. The structure-activity relationships of the 11 compounds, including their synthetic analogs, revealed the significance of the hydroquinone moiety in the anti-inflammatory activity of these sesquiterpenoids in RAW264 cells. Diacetylated zonarol (9) exhibited an activity comparable to that of zonarol as a result of intracellular deacetylation. These results provide new insights into the anti-inflammatory activity of hydroquinone-containing natural products.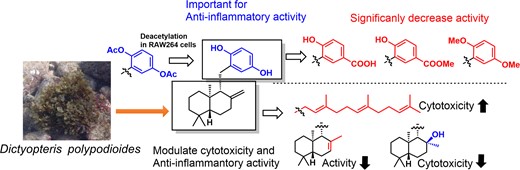
ABSTRACT: The red algal genus Portieria is a prolific producer of halogenated monoterpenoids. In this study, we isolated and characterised monoterpenoids from the Okinawan red algae Portieria hornemannii. A new polyhalogenated cyclic monoterpenoid, 2(R)-chloro-1,6(S)-dibromo-3(8)(Z)-ochtoden-4(R)-ol (1), along with three known monoterpenoids, (2R,3(8)E,4S,6R)-6-bromo-2-chloro-1,4-oxido-3(8)-ochtodene (2), 1-bromo-2-chloroochtoda-3(8),5-dien-4-one (3), and 2-chloro-1-hydroxyochtoda-3(8),5-dien-4-one (4) were isolated from the methanol extract of three populations of P. hornemannii. These compounds were characterised using a combination of spectroscopic methods and chemical synthesis, and the absolute stereochemistry of compounds 1 and 2 was determined. In addition, all isolated compounds were screened for their anti-biofouling activity against the mussel Mytilus galloprovincialis, and 1 exhibited strong activity. Therefore, halogenated monoterpenoids have the potential to be used as natural anti-biofouling drugs. 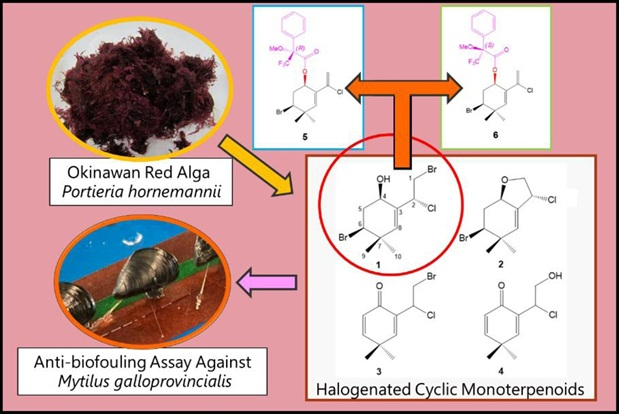
ABSTRACT: Histrionicotoxin (HTX) alkaloids, which are isolated from Colombian poison dart frogs, are analgesic neurotoxins that modulate nicotinic acetylcholine receptors (nAChRs) as antagonists. Perhydrohistrionicotoxin (pHTX) is the potent synthetic analogue of HTX and possesses a 1-azaspiro[5.5]undecane skeleton common to the HTX family. Here, we show for the first time the divergent nine-step synthesis of pHTX and its three stereoisomers from the known aldehyde through a one-step construction of the 1-azaspiro[5.5]undecane framework from a linear amino ynone substrate. Surprisingly, some pHTX diastereomers exhibited antagonistic activities on the chicken α4β2-neuronal nAChRs that were more potent than pHTX.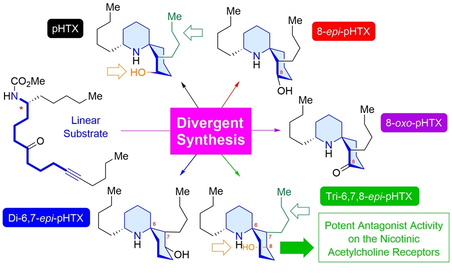
2023 年 ( 令和 5 年 )
ABSTRACT: Biomimetic epoxide-opening cascade cyclizations of polyepoxides enable the efficient and rapid construction of polyether skeletons. In this study, we discovered a method for switching the cyclization mode from tetrahydrofuran to tetrahydropyran (THP) formation in epoxide-opening cascades of polyepoxides. The THP formation proceeded via an epoxonium-ion intermediate by simple heating in neutral water. Next, by expanding the switching reaction, we successfully established a “ring-size-divergent” synthetic strategy that enabled the synthesis of the five-, six-, and seven-membered ether rings from identical diepoxide cyclization precursors under simple acidic or neutral conditions. The “ring-size-divergent” synthetic strategy was applied to the short divergent synthesis of nerolidol-type sesquiterpenoids and feroniellins, resulting in the revision of the proposed stereochemistry of certain natural products and the determination of all of the absolute configurations. Additionally, the anti-inflammatory activities of the synthetic samples were evaluated. 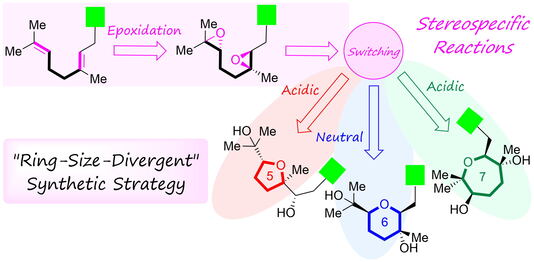
"Two New Eremophilane-Type Sesquiterpenoids from Japanese Liverwort Bazzania japonica" R. Fukada, J. Kawano, T. Tsuruta, T. Nonaka, K. Sato, S. Miyazawa, S. Ishigami, T. Ishii, K. Nishikawa, Y. Asakawa, and T. Kamada Chem. Biodiversity 20, e20230013 (2023).
Selected as Front Cover.
ABSTRACT: Two new eremophilane-type sesquiterpenoids, fusumaols A (1) and B (2), were isolated from the stem-leafy liverwort, Bazzania japonica collected in Mori-Machi, Shizuoka, Japan. Their structures were established using extensive spectroscopic (IR, MS, and 2D NMR) data, and the absolute configuration of 1 was determined by the modified Mosher's method. This is the first time eremophilanes have been discovered in the liverwort genus Bazzania. Compounds 1 and 2 were evaluated for their repellent activity against the adult population of the rice weevil Sitophilus zeamais using the modified filter paper impregnation method. Both sesquiterpenoids showed moderate repellent activities.

2022 年 ( 令和 5 年 )
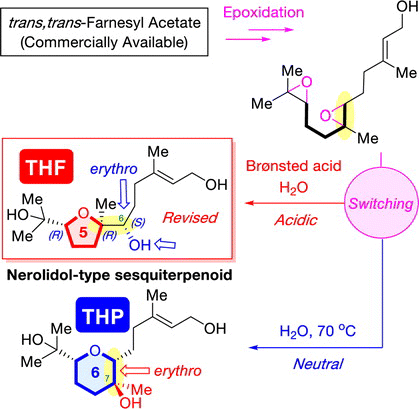
ABSTRACT: A divergent asymmetric synthesis of two nerolidol-type sesquiterpenoids with a five- or a six-membered ether ring was established from an identical commercially available trans,trans-farnesyl acetate through the cyclization of diepoxide precursors under simple acidic or neutral conditions, respectively. In addition, the relative configuration of a nerolidol-type sesquiterpenoid with a tetrahydrofuran ring was revised. Its absolute configuration was determined by its asymmetric synthesis and a modified Mosher’s analysis. Furthermore, the cytotoxicity and nitric oxide production inhibitory activity of the synthesized compounds were assessed.
"Total Synthesis, Revised Structure, and Cytotoxic Activities of Iubol" K. Nishikawa, N. Taki, K. Nishikibe, M. Kumagai, and Y. Morimoto Chem. Lett. 51, 1000-1003 (2022).
Selected as Front Cover.
ABSTRACT: To unambiguously establish the stereostructure of the marine cytotoxic bromotriterpenoid iubol, a member of the thyrsiferol family, asymmetric chemical synthesis has been carried out. The synthesis features a one-pot process for the tetrahydropyranyl D ring construction through a stoichiometric Sharpless asymmetric epoxidation of allylic alcohols followed by titanium chelation-assisted 6-exo oxacyclization. In this paper, we report the asymmetric total synthesis of iubol, revision of the proposed structure to the C22-epimer, and preliminary cytotoxic activities of synthetic compounds against some tumor cells.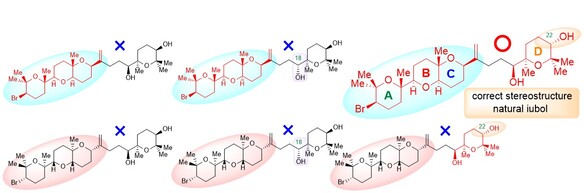

ABSTRACT: Sequestration of chemical defenses from dietary sources is dependent on the availability of compounds in the environment and the mechanism of sequestration. Previous experiments have shown that sequestration efficiency varies among alkaloids in poison frogs, but little is known about the underlying mechanism. The aim of this study was to quantify the extent to which alkaloid sequestration and modification are dependent on alkaloid availability and/or sequestration mechanism. To do this, we administered different doses of histrionicotoxin (HTX) 235A and decahydroquinoline (DHQ) to captive-bred Adelphobates galactonotus and measured alkaloid quantity in muscle, kidney, liver, and feces. HTX 235A and DHQ were detected in all organs, whereas only DHQ was present in trace amounts in feces. For both liver and skin, the quantity of alkaloid accumulated increased at higher doses for both alkaloids. Accumulation efficiency in the skin increased at higher doses for HTX 235A but remained constant for DHQ. In contrast, the efficiency of HTX 235A accumulation in the liver was inversely related to dose and a similar, albeit statistically nonsignificant, pattern was observed for DHQ. We identified and quantified the N-methylation of DHQ in A. galactonotus, which represents a previously unknown example of alkaloid modification in poison frogs. Our study suggests that variation in alkaloid composition among individuals and species can result from differences in sequestration efficiency related to the type and amount of alkaloids available in the environment.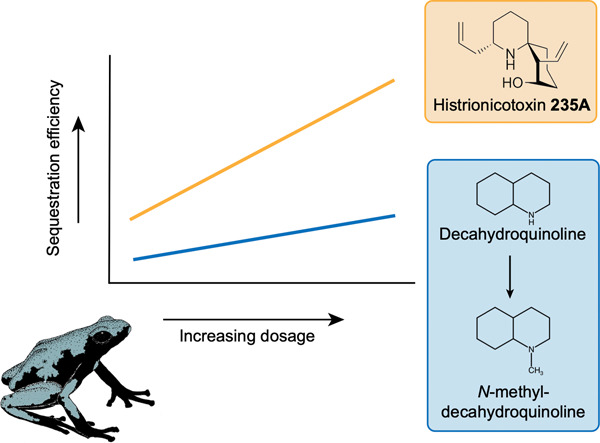
"Asymmetric Total Synthesis of Toxicodenane A by Samarium-Iodide-Induced Barbier-Type Cyclization and Its Cell-Protective Effect against Lipotoxicity" K. Nishikawa, K. Kikuta, T. Tsuruta, H. Nakatsukasa, S. Sugahara, S. Kume, and Y. Morimoto Org. Lett. 24, 531-535 (2022).
Selected as Supplementary Cover Art.
Highlighted in Synfacts 18, 0441 (2022)
ABSTRACT: The asymmetric total synthesis of toxicodenane A, a sesquiterpenoid expected to be promising for diabetic nephropathy, was achieved. In the synthesis, a samarium iodide (SmI2)-induced Barbier-type cyclization and a regio- and stereoselective allylic oxidation followed by a dehydration cyclization were employed as key steps. Furthermore, the first asymmetric syntheses of both enantiomers were accomplished using the previously mentioned synthetic strategy. Finally, the synthetic compounds significantly inhibited lipotoxicity-mediated inflammatory and fibrotic responses in mouse renal proximal tubular cells.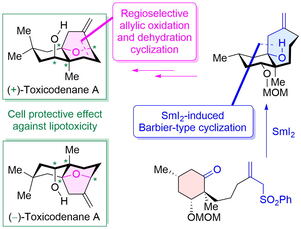

"Asymmetric Total Syntheses, Stereostructures, and Cytotoxicities of Marine Bromotriterpenoids Aplysiol B (Laurenmariannol) and Saiyacenol A" K. Nishikibe, K. Nishikawa, M. Kumagai, M. Doe, and Y. Morimoto Chem. Asian J. 17, e202101137 (2022) [ Open Access ].
Selected as Front Cover.
ABSTRACT: Total assignments of stereostructures of marine cytotoxic bromotriterpenoids, aplysiol B (laurenmariannol) and saiyacenol A, were accomplished through their asymmetric chemical syntheses to elucidate their ambiguous stereostructures and whether or not other members showing the opposite chirality for the ABC ring system exist. Moreover, their preliminary growth inhibitory activities against some tumor cells were also evaluated.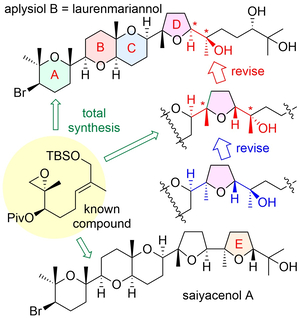

ABSTRACT: One new furanocembranoid diterpene, 11-hydroxy-Δ12(13)-pukalide (1), along with six known secondary metabolites, 11-acetoxy-Δ12(13)-pukalide (2), 13α-acetoxypukalide (3), pukalide (4), 3α-methoxyfuranocembranoid (5), Δ9(15)-africanene (6), and methyl (5′E)-5-(2′,6′-dimethylocta-5′,7′-dienyl)furan-3-carboxylate (7) were isolated from the Okinawan soft coral Sinularia sp. Their chemical structures were elucidated based on spectroscopic analysis (FTIR, NMR, and HRESIMS), and the relative stereochemistry of 1 was determined by NOESY experiments and acetylation, which yielded derivative 2. In addition, compounds 1 and 7 exhibited toxicity in the brine shrimp lethality test.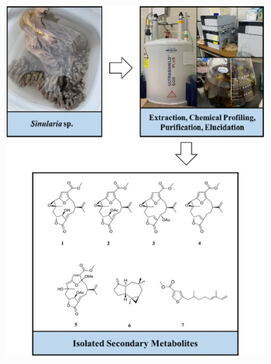
2021 年 ( 令和 4 年 )
"Establishing a “Ring Size-Divergent” Synthetic Strategy: Synthesis, Structural Revision, and Absolute Configuration of Feroniellins" K. Nishikawa, T. Niwa, K. Nishikibe, M. Kumagai, and Y. Morimoto Chem. Eur. J. 27, 11045–11049 (2021).
ABSTRACT: A “ring-size-divergent” strategy enabled us to divergently synthesize the five-, six-, and seven-membered ether rings of feroniellin analogs from diepoxides under simple acidic or neutral conditions in only two steps from bergamottin. Additionally, the proposed structures of feroniellins A and B were revised, and the absolute configurations of all feroniellins were determined from their asymmetric synthesis.
ABSTRACT: A one-step synthesis of the 1-azaspiro[5.5]undecane skeleton in histrionicotoxin alkaloids from a linear substrate was realized utilizing a Hg(OTf)2-catalyzed cycloisomerization reaction. Histrionicotoxin alkaloids as potential target drugs were successfully synthesized via our developed cyclization.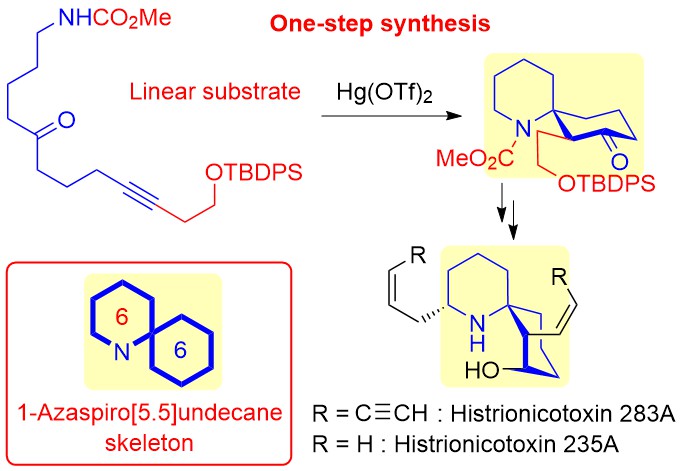
ABSTRACT: In this contribution, we propose a new synthetic approach to tetrodotoxin (TTX), one of the most famous marine toxins that, after first preparing a functionalized linear substrate, forms a cyclohexane core from the substrate utilizing our mercuric triflate (Hg(OTf)2)-catalyzed cycloisomerization reaction. The concept was applied to the synthesis of 11-nor-6,7,8-trideoxyTTX and 11-nor-4,9-anhydro-6,7,8-trideoxyTTX, which are unnatural TTX analogues, demonstrating the validity of our new approach.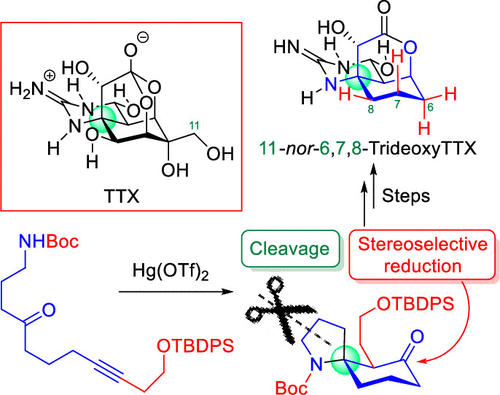
ABSTRACT: Our concept of natural product synthesis is to directly construct the ring skeletons, frequently occurring in natural products with strong biological activities, from easily accessible linear substrates, and our group has established practical methods for synthesizing useful natural products along our synthetic strategy. First, we developed a cycloisomerization reaction to directly synthesize nitrogen-containing spirocycles from linear substrates, accompanied with construction of a stereogenic tetrasubstituted spiro-carbon. The reaction enabled us to achieve the efficient total synthesis of lepadiformines, revealing a phenomenon of their enantiodivergence. The synthesis of histrionicotoxins, frog poisons, was also accomplished through our original cyclization reaction. In addition, we found the critical switching of cyclization modes of polyepoxides in acidic aqueous media and neutral water, and the novel cascade cyclization was applied to the synthesis of natural products.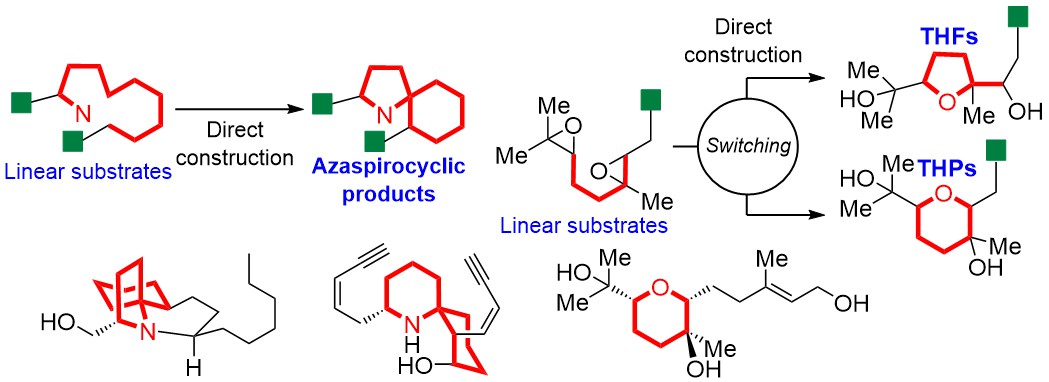
ABSTRACT: Obesity is a risk factor for many diseases, including type 2 diabetes and cardiovascular disease, and is related to the rising morbidity and mortality. Discovery of agents targeting adipogenesis, especially from natural sources, is important for the treatment of obesity. Here, we aimed to identify anti-adipogenic substances in methanol extracts of Physalis peruviana and to investigate their effect, along with underlying mechanisms. Activity-guided fractionation of the extract revealed 4β-hydroxywithanolide E (HWE) and withanolide E (WE) as the adipogenesis inhibitors. Both compounds suppressed mRNA expression of central adipogenic transcription factors, peroxisome proliferator-activated receptor γ, and CCAAT/enhancer-binding protein α in the early stage of adipocyte differentiation. The inhibitory action of these two withanolides on adipogenesis was largely limited to this stage. The proliferation of preadipocytes was markedly suppressed by treatment with HWE and WE for 24 and 48 h in the differentiation medium, and cell-cycle arrest in the G0/G1 phase was observed. Therefore, our results suggested that withanolides from P. peruviana to be novel anti-adipogenic compounds that modulate mitotic clonal expansion.
2020 年以前
ABSTRACT: Ambient mass spectrometry is useful for analyzing compounds that would be affected by other chemical procedures. Poison frogs are known to sequester alkaloids from their diet, but the sequestration pathway is unknown. Here, we describe methods for whole-body cryosectioning of frogs and use desorption electrospray ionization mass spectrometry imaging (DESI-MSI) to map the orally administered alkaloid histrionicotoxin 235A in a whole-body section of the poison frog Dendrobates tinctorius. Our results show that whole-body cryosectioning coupled with histochemical staining and DESI-MSI is an effective technique to visualize alkaloid distribution and help elucidate the mechanisms involved in alkaloid sequestration in poison frogs.
"Fluorinated Kavalactone Inhibited RANKL-Induced Osteoclast Differentiation of RAW264 Cells" M. Kumagai, K. Nishikawa, T. Mishima, I. Yoshida, M. Ide, A. Watanabe, K. Fujita, and Y. Morimoto Biol. Pharm. Bull. 43, 898–903 (2020) [ Open Access ].
ABSTRACT: Bone loss and bone-related disease are associated with the deregulation of osteoclast function, and therefore agents that affect osteoclastogenesis have attracted attention. The purpose of the present study was to discover modified kavalactone analogs as potential anti-osteoclastogenic agents. We assessed the effect of 26 analogs on osteoclast differentiation in vitro. The most potent compound, (E)-6-(2-fluorostyryl)-4-methoxy-2H-pyran-2-one (22), suppressed receptor activator of nuclear factor-κB ligand (RANKL)-induced osteoclastogenic differentiation of RAW264 cells with IC50 values of 4.3 M. A partial structure–activity relationship study revealed the importance of fluorine and its position within the 5,6-dehydrokawain skeleton. The results of a pit formation assay suggested that compound 22 prevents osteoclastic bone resorption by inhibiting osteoclastogenesis. Moreover, compound 22 downregulated mRNA expression levels of RANKL-induced nuclear factor of activated T cells c1 (NFATc1) and osteoclastogenesis-related genes. These results suggest that (E)-6-(2-fluorostyryl)-4-methoxy-2H-pyran-2-one scaffold could lead to the identification of new anti-resorptive agents.
"Critical Switching of Cyclization Modes of Polyepoxides in Acidic Aqueous Media and Neutral Water: Synthesis and Revised Structure of a Nerolidol‐Type Sesquiterpenoid" K. Nishikawa, K. Morita, S. Hashimoto, A. Hoshino, T. Ikeuchi, M. Kumagai, and Y. Morimoto Angew. Chem. Int. Ed. 58, 10168–10172 (2019);
Angew. Chem. 131, 10274–10278 (2019).
Selected as Front Cover.
ABSTRACT: Biomimetic epoxide-opening cascades of polyepoxides enable the efficient and rapid construction of polyether frameworks. Herein, we show that the epoxide-opening cascade cyclization that affords tetrahydrofuran products in acidic aqueous media produces tetrahydropyran (THP) in neutral water. THP formation proceeded by simply heating polyepoxides in neutral water and followed a different cyclization mode from those observed so far. The novel cascade cyclization in H2O was applied to the synthesis of a new nerolidol-type sesquiterpenoid, resulting in revision of the proposed structure and determination of the absolute configuration.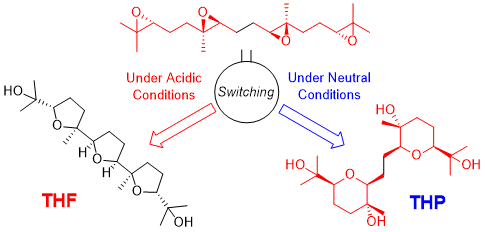

“Formal Total Synthesis of Histrionicotoxin Alkaloids via Hg(OTf)2-catalyzed Cycloisomerization and SmI2-induced Ring Expansion” K. Matsumura, K. Nishikawa, H. Yoshida, M. Doe, and Y. Morimoto RSC Adv. 8, 11296-11303 (2018) [ Open Access ].
ABSTRACT: The efficient formal total synthesis of histrionicotoxin alkaloids was achieved. In this process, two key reactions were used to construct a core 1-azaspiro[5.5]undecane framework common to histrionicotoxins: a mercuric triflate (Hg(OTf)2)-catalyzed cycloisomerization of a linear substrate, which was developed in our laboratory, and a samarium iodide (SmI2)-mediated ring expansion.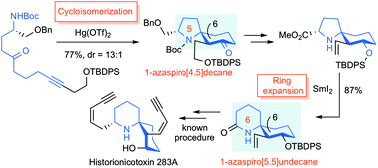
"Evaluation of Aculeatin and Toddaculin Isolated from Toddalia asiatica as Anti-inflammatory Agents in LPS-Stimulated RAW264 Macrophages" M. Kumagai, A. Watanabe, I. Yoshida, T. Mishima, M. Nakamura, K. Nishikawa, and Y. Morimoto Biol. Pharm. Bull. 41, 132-137 (2018) [ Open Access ].
ABSTRCT: Anti-inflammatory activity of aculeatin and toddaculin, which are coumarins with a similar structure isolated from Toddalia asiatica (L.) LAM., was evaluated using lipopolysaccharide (LPS)-stimulated RAW264 mouse macrophage cells. Both aculeatin and toddaculin significantly inhibited mRNA expression of inflammatory mediators and nitric oxide production. Furthermore, Toddaculin suppressed LPS-induced phosphorylation of p38 and extracellular signal-regulated kinase (ERK)1/2 and inhibited LPS-induced activation of nuclear factor-kappaB (NF-κB). However, aculeatin did not exhibit such effects, suggesting that aculeatin and toddaculin suppress LPS-induced inflammation of RAW264 cells via different mechanisms. The cellular uptake of these compounds was also evaluated. Toddaculin was detected in RAW264 cells after 4 and 24 h. However, aculeatin levels were not observed in RAW264 cells at all incubation intervals. These results indicate that de-epoxidation of a prenyl group can increase hydrophobicity of molecule and is thought to accelerate cellular uptake and/or interactions with the phospholipid bilayers of cell membranes.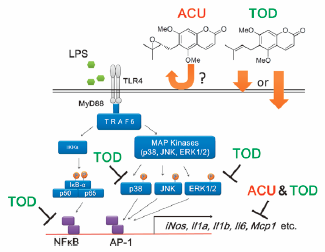
"Total Syntheses of Lepadiformine Marine Alkaloids with Enantiodivergency, Utilizing Hg(OTf)₂-Catalyzed Cycloisomerization Reaction and Their Cytotoxic Activities" K. Nishikawa, K. Yamauchi, S. Kikuchi, S. Ezaki, T. Koyama, H. Nokubo, K. Matsumura, T. Kodama, M. Kumagai, and Y. Morimoto Chem. Eur. J. 23, 9535-9545 (2017).
Selected as Back Cover.
ABSTRACT: A phenomenon of enantiodivergence was found in lepadiformine alkaloids isolated from a single species marine tunicate Clavelina moluccensis through their syntheses. The enantioselective total syntheses have been achieved by a key mercury(II) triflate-catalyzed cycloisomerization reaction developed in our laboratory (see scheme), and cytotoxic activities of synthesized compounds were also evaluated.

"Synthesis of Novel 5,6-Dehydrokawain Analogs as Osteogenic Inducers and Their Action Mechanisms" M. Kumagaia, K. Nishikawa, T. Mishima, I. Yoshida, M. Ide, K. Koizumi, M. Nakamura, and Y. Morimoto Bioorg. Med. Chem. Lett. 27, 2401–2406 (2017).
ABSTRACT: An imbalance between bone resorption by osteoclasts and bone formation by osteoblasts can cause bone loss and bone-related disease. In a previous search for natural products that increase osteogenic activity, we found that 5,6-dehydrokawain (1) from Alpinia zerumbet promotes osteoblastogenesis. In this study, we synthesized and evaluated series of 5,6-dehydrokawain analogs. Our structure-activity relationships revealed that alkylation of para or meta position of aromatic ring of 1 promote osteogenic activity. Among the potential analogs we synthesized, (E)-6-(4-Ethylstyryl)-4-methoxy-2H-pyran-2-one (14) and (E)-6-(4-Butylstyryl)-4-methoxy-2H-pyran-2-one (21) both significantly up-regulated Runx2 and Osterix mRNA expression at 10 M. These osteogenic activities could be mediated by bone morphogenetic protein (BMP) and activation of p38 MAPK signaling pathways. Compounds 14 and 21 also inhibited RANKL-induced osteoclast differentiation of RAW264 cells. These results indicated that novel 5,6-dehydrokawain analogs not only increase osteogenic activity but also inhibit osteoclast differentiation, and could be potential lead compounds for the development of anti-osteoporosis agents.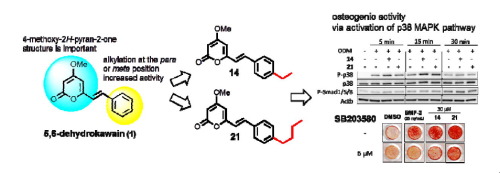
"Total Synthesis of the Cytotoxic Marine Triterpenoid Isodehydrothyrsiferol Reveals Partial Enantiodivergency in the Thyrsiferol Family of Natural Products" A. Hoshino, H. Nakai, M. Morino, K. Nishikawa, T. Kodama, K. Nishikibe, and Y. Morimoto Angew. Chem. Int. Ed. 56, 3064-3068 (2017);
Angew. Chem. 129, 3110–3114 (2017).
ABSTRACT: Enantioselective total synthesis of the cytotoxic marine triterpenoid isodehydrothyrsiferol revealed partial enantiodivergence in that the ABC ring system is enantiomeric to that of the other members of this natural product family. Furthermore, this unprecedented partial enantiodivergence is observed between dehydrothyrsiferol and isodehydrothyrsiferol, which originate from a single species, the red alga Laurencia viridis. 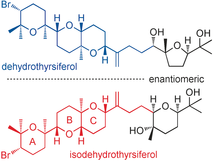
"Total Synthesis of Nitropyrrolins A, B, and D" H. Mitani, T. Matsuo, T. Kodama, K. Nishikawa, Y. Tachi, and Y. Morimoto Tetrahedron 72, 7179–7184 (2016).
ABSTRACT: The chemical synthetic method of cytotoxic nitropyrrolins A (1), B (2), and D (4), 2-nitropyrrole terpenoids rarely occurring in nature, has been developed for further biological studies. After the synthesis of nitropyrrolin B has first been achieved, nitropyrrolin B was transformed into nitropyrrolins A and D in two steps and one step, respectively. The regio- and stereoselective epoxide-cleavage reaction was highlighted in the direct conversion of nitropyrrolin B to D. 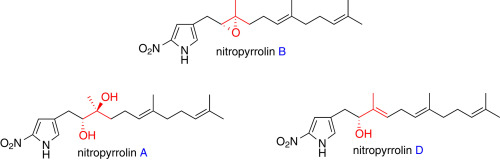
"5,6-Dehydrokawain from Alpinia zerumbet Promotes Osteoblastic MC3T3-E1 Cell Differentiation" M. Kumagai, T. Mishima, A. Watanabe, T. Harada, I. Yoshida, K. Fujita, M. Watai, S. Tawata, K. Nishikawa, and Y. Morimoto Biosci. Biotech. Biochem. 80, 1425-1432 (2016) [ Open Access ].
ABSTRACT: Bone homeostasis is maintained by balancing bone formation and bone resorption, but an imbalance between them is associated with various bone-related diseases such as osteoporosis and rheumatoid arthritis. We found that 5,6-dehydrokawain (DK) and dihydro-5,6-dehydrokawain (DDK), which were isolated aspromising compounds from Alpinia zerumbet rhizomes, promote differentiation of osteoblastic MC3T3-E1 cells. DK and DDK increased the alkaline phosphatase activity and matrix mineralization of MC3T3-E1 cells. DK exerts larger effects than DDK. The gene expression of runt-related transcription factor 2 and osterix, which are essential transcription factors in the early period of osteoblast differentiation, was significantly increased by DK treatment. The mRNA level of distal-less homeobox 5 was also enhanced by DK treatment, and DK activated the p38 mitogen-activated protein kinase pathway. Therefore, DK may have clinical potential for preventing osteoporosis, and could be considered as a potential anabolic therapeutic agent. 5,6-Dehydrokawain (DK) and dihydro-5,6-dehydrokawain (DDK) from Alpinia zerumbet rhizomes, promote differentiation of osteoblastic MC3T3-E1 cells. 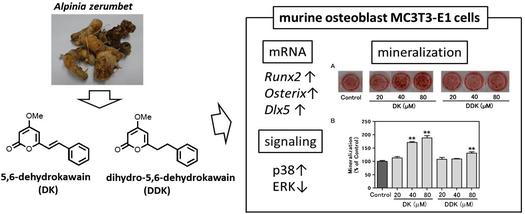
"Total Synthesis of (−)-Lepadiformine A Utilizing Hg(OTf)2-Catalyzed Cycloisomerization Reaction" K. Nishikawa, S. Kikuchi, S. Ezaki, T. Koyama, H. Nokubo, T. Kodama, Y. Tachi, and Y. Morimoto Org. Lett. 17, 5772-5775 (2015);
Highlighted in Synfacts 2, 116 (2016).
ABSTRACT: A cytotoxic marine alkaloid (−)-lepadiformine A (1) possesses a unique structure characterized by the trans-1-azadecalin AB ring system fused with the AC spiro-cyclic ring. In this research, we found that a cycloisomerization reaction from amino ynone 2 to a 1-azaspiro[4.5]decane skeleton 3, corresponding to the AC ring system of 1, is promoted by Hg(OTf)2. Thus, we have accomplished the efficient total synthesis of (−)-lepadiformine A in 28% overall yield by featuring the novel Hg(OTf)2-catalyzed cycloisomerization. 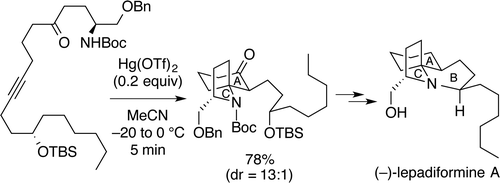
"Total Synthesis and Complete Stereochemical Assignment of Heronapyrroles A and B" T. Matsuo, S. Hashimoto, K. Nishikawa, T. Kodama, S. Kikuchi, Y. Tachi, Y. Morimoto Tetrahedron Lett. 56, 5345-5348 (2015).
ABSTRCT: Heronapyrroles A and B with promising and selective antibacterial activity but no cytotoxicity against mammalian cell lines are among members of rare and unique 4-farnesylated-2-nitropyrrole natural products. The absolute configurations at C7 and C15 have been proposed to be 7S and 15R by the modified Mosher method. In this Letter, we have achieved the total synthesis of natural (+)-heronapyrroles A (1) and B (2) and report that the correct absolute configurations are 7R, 8S, and 15S as shown in the structural formulas 1 and 2. 
"Biomimetic Total Synthesis of (-)-Neroplofurol and (+)-Ekeberin D4 Triggered by Hydrolysis of Terminal Epoxides" T. Kodama, S. Aoki, T. Matsuo, Y. Tachi, K. Nishikawa, and Y. Morimoto Chem. Lett. 43, 1662-1664 (2014).
ABSTRACT: To accumulate the chemical basis of epoxide-opening cascade biogenesis, chemical syntheses of sesqui- and triterpenoids were performed. The biomimetic total syntheses of (−)-neroplofurol (1) and (+)-ekeberin D4 (2) were accomplished by protic acid-catalyzed hydrolysis of the terminal epoxide from nerolidol diepoxide 3 and squalene tetraepoxide 4 through single and double 5-exo cyclizations in intermediates 5 and 6, respectively. This chemical reaction mimics the direct hydrolysis mechanism of epoxide hydrolases, enzymes that catalyze an epoxide-opening reaction to finally produce vicinal diols. 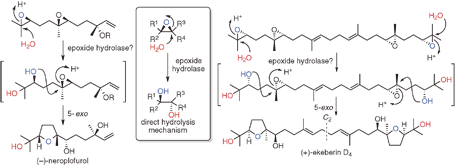
"Analysis of Enantiofacial Selective Epoxidation Catalyzed by Flavin-Containing Monooxygenase Lsd18 Involved in Ionophore Polyether Lasalocid Biosynthesis" G. Suzuki, A. Minami, M. Shimaya, T. Kodama, Y. Morimoto, H. Oguri, and H. Oikawa Chem. Lett. 43, 1779-1781 (2014).
ABSTRACT: Enzymatic epoxidation represents a key biosynthetic transformation in the construction of polyether skeletons. A single flavin-containing monooxygenase, Lsd18, is involved in ionophore polyether lasalocid biosynthesis and participates in the enantioselective epoxidations of the diene precursor. Biotransformation studies utilizing structurally simplified monoolefin analogs with different substitution patterns revealed important structural requirements for the enantiofacial selectivity of Lsd18-catalyzed epoxidations. These results enabled us to propose a substrate binding model of Lsd18, which was applied to the biosynthesis of other polyethers. 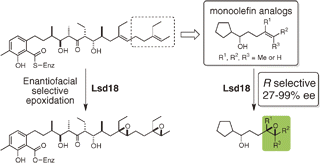
"A Convergent Total Synthesis of Antiplasmodial C2 Symmetric (+)-Ekeberin D4" T. Kodama, S. Aoki, S. Kikuchi, T. Matsuo, Y. Tachi, K. Nishikawa, and Y. Morimoto Tetrahedron Lett. 54, 5647-5649 (2013).
ABSTRACT: The first concise total synthesis of C2 symmetric (+)-ekeberin D4 (1) that exhibits antiplasmodial activity has been achieved in total nine steps and 27% yield from the known diol 4. The efficient synthetic method features the regio- and diastereoselective epoxidation of 4 and convergent coupling between half fragments 2 and 3 by taking into account the C2 symmetric property. 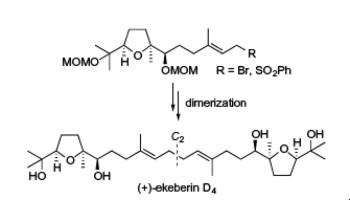
"Biomimetic Epoxide-Opening Cascades of Oxasqualenoids Triggered by Hydrolysis of the Terminal Epoxide" Y. Morimoto, E. Takeuchi, H. Kambara, T. Kodama, Y. Tachi, and K. Nishikawa Org. Lett. 15, 2966–2969 (2013).
ABSTRACT: The biomimetic epoxide-opening cascades from squalene polyepoxides 4–6 to triterpene polyethers (oxasqualenoids) teurilene (1), glabrescol (2), and omaezakianol (3), respectively, were reproduced in a single event by chemical reaction. These cascades proceeded through the 5-exo tandem cyclization triggered by Brønsted acid-catalyzed hydrolysis of the terminal epoxide, mimicking the direct hydrolysis mechanism of epoxide hydrolases. 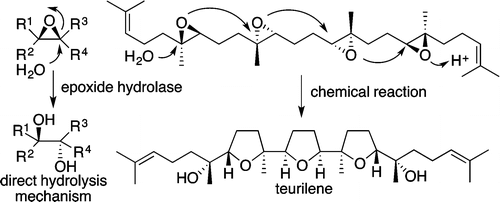
"TIPSOTf-promoted Tandem Reaction through Rearrangement of Epoxides into Aldehydes with Selective Alkyl Migration Followed by Prins-Type Cyclization to Cyclopentanes" T. Kodama, S. Harada, T. Tanaka, Y. Tachi, and Y. Morimoto Synlett 3, 458-462 (2012)
ABSTRACT: The tandem reaction of trisubstituted epoxides to cyclopentanes promoted by TIPSOTf in nitromethane has been found. It consists of stereospecific rearrangement of epoxides into aldehydes accompanied with selective alkyl migration and subsequent Prins-type cyclization of the aldehydes generated to cyclopentanes. 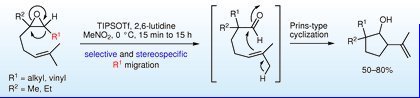
"Total Synthesis of Marine Halogen-Containing Triterpene Polyethers Using Regioselective 5-exo and 6-endo Cyclizations and the Stereochemistry" Y. Morimoto J. Synth. Org. Chem., Jpn. 70, 154-165 (2012).
ABSTRACT: Aurilol (1), intricatetraol (3), and enshuol (5), marine halogen-containing members of a family of squalene-derived triterpene polyethers named oxasqualenoids, were isolated from the sea hare Dolabella auricularia, the red alga Laurencia inricata, and omaezakiana Masuda, respectively. Although their planar structures and partial configurations were elucidated by spectroscopic and chemical analyses, until now their entire configurations had not been determined. Many other types of oxasqualenoids have also been isolated from both marine and terrestrial organisms; however, it is often difficult to determine their stereostructures even by modern highly advanced spectroscopic methods, especially in the case of acyclic systems that include stereogenic quaternary carbon centers. Such systems expose the technical limitations of the current highly advanced NMR spectroscopic methods used for the structural elucidation of diverse and complex natural products. Herein, we report the total assignments of the previously incomplete stereostructures of 1, 3, and 5 to 2, 4, and 6, respectively, through the first asymmetric total syntheses featuring regioselective 5-exo and 6-endo cyclizations of bishomoepoxy alcohols.
“Diastereoselective Synthesis of the Indeno-tetrahydropyridine Core Bearing a Diaryl-substituted Stereogenic Quaternary Carbon Center of Haouamine B” T. Tanaka, H. Inui, H. Kida, T. Kodama, T. Okamoto, A. Takeshima, Y. Tachi and Y. Morimoto Chem. Commun. 47, 2949-2951 (2011).
ABSTRACT: The characteristic indeno-tetrahydropyridine core of cytotoxic haouamine B (2) was efficiently synthesized featuring the diastereoselective construction of a diaryl-substituted stereogenic quaternary center by an intramolecular Pd-catalyzed α-C-arylation and subsequent direct conversion of the vinylogous imide function into the C2–C25 double bond by TsNHNH2. 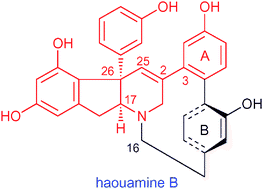
"Rings D-seco and B,D-seco Tetranortriterpenoids from Root Bark of Entandrophragma Angolense" T. K. Nsiama, H. Okamura, T. Hamada, Y. Morimoto, M. Doe, T. Iwagawa, and M. Nakatani Phytochemistry 72, 1854-1858 (2011).
“Cytotoxic Isomalabaricane Derivatives and a Monocyclic Triterpene Glycoside from the Sponge Rhabdastrella Globostellata” M. Hirashima, K.Tsuda, T. Hamada, H. Okamura, T. Furukawa, S. Akiyama, Y. Tajitsu, R. Ikeda, M. Komatsu, M. Doe, Y. Morimoto, M. Shiro, R. W. M. van Soest, K. Takemura, and T. Iwagawa J. Nat. Prod. 73, 1512-1518 (2010).
“Cytotoxic Biscembranes from the Soft Coral Saricophyton glaucum” T. Iwagawa, K. Hashimoto, Y. Yokogawa, H. Okamura, M. Nakatani, M. Doe, Y. Morimoto and K. Takemura J. Nat. Prod. 72, 946-949 (2009).
“Total Synthesis and Determination of the Absolute Configuration of (+)-Omaezakianol ” Y. Morimoto, T. Okita and H. Kambara Angew. Chem., Int. Ed. 48, 2538-2541 (2009).
Angew. Chem. 121, 2576-2579 (2009).
ABSTRACT: The first asymmetric total synthesis of the marine tetracyclic oxasqualenoid (+)-omaezakianol features a convergent olefin cross-metathesis between a monotetrahydrofuran fragment and a triepoxy alkene, and cascade oxacyclizations of a triepoxy alcohol to form the right-hand three ether rings. The total synthesis proved the absolute configuration of (+)-omaezakianol to be that shown.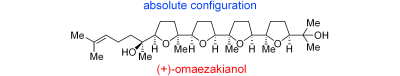
“Squalene-Derived Triterpene Polyethers from the Red Alga Laurencia Omaezakiana” Y. Matsuo, M. Suzuki, M. Masuda, T. Iwai and Y. Morimoto Helv. Chim. Acta 91, 1261-1266 (2008).
ABSTRACT: In our continuing search on halogenated metabolites from species of the red algal genus Laurencia, a novel squalene-derived triterpene polyether, named omaezakianol (2), was isolated from Laurencia omaezakiana Masuda along with 15,16-anhydrothyrsiferol (3). Their structures were determined by spectral and chemical methods.
“Limonoids from the Stem Bark of Cedrela Odorata” N. T. Kipassa, T. Iwagawa, H. Okamura, M. Doe, Y. Morimoto, and M. Nakatani Photochemistry 69, 1782-1787 (2008).
“The Role of Chemical Synthesis in Structure Elucidation of Oxasqualenoids” Y. Morimoto Org. Biomol. Chem. 6, 1709-1719 (2008).
ABSTRACT: Recently, highly oxidized and structurally diverse triterpene polyethers, which are thought to be biogenetically squalene-derived natural products (oxasqualenoids), have been isolated from both marine and terrestrial organisms. However, it is often difficult to determine their stereostructures even by the current, highly advanced spectroscopic methods, especially in acyclic systems including stereogenic quaternary carbon centers. In such cases, it is effective to predict and synthesize the possible stereostructures. Herein, we report total assignments of the previously incomplete stereostructures of an epoxy tri-THF diol, intricatetraol and enshuol, members of the oxasqualenoids, through the first asymmetric total syntheses of the natural products, the configurations of which are difficult to determine by other means. Since this article is basically written as a communication without detailed experimental procedures and spectroscopic data, original papers with full data should follow..gif)
“Three Mexicanolides from the Root Bark of Entandrophragma Angolense” T. N. Kipassa, H. Okamura, M. Doe, Y. Morimoto, T. Iwagawa, and M. Nakatani Heterocycles 75, 157-164 (2008).
“Assignment of the Absolute Configuration of the Marine Pentacyclic Polyether (+)-Enshuol by Total Synthesis” Y. Morimoto, H. Yata, and Y. Nishikawa Angew. Chem., Int. Ed. 46, 6481-6484 (2007);
Angew. Chem. 119, 6601-6604 (2007).
ABSTRACT: The complete stereostructure of the marine pentacyclic triterpene polyether (+)-enshuol is shown. Asymmetric total synthesis confirmed the configuration predicted on the basis of NMR spectroscopic data of the previously synthesized natural products aurilol and glabrescol, substructures of which are present in enshuol, and disproved an earlier prediction based on biogenetic considerations. .gif)
“Total Synthesis and Determination of the Absolute Configuration of (+)-Intricatetraol” Y. Morimoto, T. Okita, M. Takaishi, and T. Tanaka Angew. Chem., Int. Ed. 46, 1132-1135 (2007);
Angew. Chem. 119, 1150-1153 (2007).
ABSTRACT: The total synthesis of the marine triterpene polyether (+)-intricatetraol (1) has revealed its absolute configuration, which could not be determined even by spectroscopic methods. The approach features the enantioselective construction of the unique vicinal bromochloro functionality and an efficient olefin-metathesis strategy that takes the C2 symmetry of the target into consideration. .gif)
“Two-directional Synthesis and Stereochemical Assignment toward a C-2 Symmetric Oxasqualenoid (+)-Intricatetraol ” Y. Morimoto, M. Takaishi, N. Adachi, T. Okita, and H.Yata Org. Biomol. Chem. 4, 3220-3222 (2006).
ABSTRACT: The asymmetric synthesis of tetraol (+)-3, a degradation product derived from a C2 symmetric oxasqualenoid intricatetraol 1, has been achieved through the two-directional synthesis starting from diol 7, realizing the further additional assignment of the incomplete stereostructure of 1, the stereochemistry of which is difficult to determine otherwise. .gif)
“Reagent-Controlled Swiching of 5-exo to 6-endo Cyclizations in Epoxide Openings” Y. Morimoto, Y. Nishikawa, C. Ueba, and T. Tanaka Angew. Chem., Int. Ed. 45, 810-812 (2006);
Angew. Chem. 118, 824-826 (2006).
ABSTRACT: The switching of the usual 5-exo cyclizations of epoxide substrates to the 6-endo mode, which goes against Baldwin's rule, is demonstrated by treating bishomoepoxy alcohols with triisopropylsilyl triflate (TIPSOTf) in nitromethane (see scheme). This method is different to those previously reported in which elaborate modifications of the epoxide substrate are required to attain 6-endo cyclization..gif)
“Structure, Biological Activities, and Total Syntheses of 13-Hydroxy- and 13-Acetoxy-14-nordehydrocacalohastine, Novel Modified Furanoeremophilane-type Sesquiterpenes from Trichilia Cuneata” M. Doe, T. Shibue, H. Haraguchi and Y. Morimoto Org. Lett. 7, 1765-1768 (2005).
ABSTRACT: 13-Hydroxy-14-nordehydrocacalohastine (2) and 13-acetoxy-14-nordehydrocacalohastine (3), two novel modified furanoeremophilane-type sesquiterpenes isolated from Trichilia cuneata, showed inhibitory activities for membrane lipid peroxidation in mitochondria and microsomes. The first, highly convergent total syntheses of new compounds 2 and 3 have also been achieved via a palladium-mediated three-component coupling reaction between 2-iodotoluene (7), 1-penten-4-yn-3-ol (8), and diethyl ethoxymethylenemalonate (9).
“Total Synthesis and Complete Assignment of the Stereostructure of a Cytotoxic Bromotriterpene Polyether (+)-Aurilol” Y. Morimoto, Y. Nishikawa and M. Takaishi J. Am. Chem. Soc. 127, 5806-5807 (2005).
“Structure, Synthesis, and Biological Activity of 14-Methoxy-1,2-dehydrocacalol Methyl Ether, a New Modified Furanoeremophilane Type Sesquiterpene from trichilia cuneata” M. Doe, Y. Hirai, T. Kinoshita, K. Shibata, H. Haraguchi and Y. Morimoto Chem. Lett. 33, 714-715 (2004).
“Total Synthesis of Five Cacalol Families at Different Oxidation Stages, Modified Furanoeremophilane Sesquiterpenes from Cacalia and Senecio Species” Y. Hirai, M. Doe, T. Kinoshita and Y. Morimoto Chem. Lett. 33, 136-137 (2004).
“Total Synthesis and Asssignment of the Double-Bond Position and Absolute Configuration of (-)-Pyrinodemin A” Y. Morimoto, T. Iwai, S. Kitao, T. Okita and T. Shoji Org. Lett. 5, 2611-2614 (2003).
“Stereospecific and Biomimetic Synthesis of CS and C2 Symmetric 2,5-Disubstituted Tetahydrofuran Rings as Central Building Blocks of Biogenetically Intriguing Oxasqualenoids” Y. Morimoto, T. Iwai, Y. Nishikawa and T. Kinoshita Tetrahedron: Asymmetry 13, 2641-2647 (2002).
“Complete Assignment of the Stereostructure of a New Squalene-Derived Epoxy Tri-THF Diol from Spathelia glabrescens by Total Synthesis” Y. Morimoto, M. Takaishi, T. Iwai, T. Kinoshita, H. Jacobs Tetrahedron Lett. 43, 5849-5852 (2002).
“Total Synthesis and Determination of the Stereochemistry of 2-Amino-3-cyclopropylbutanoic Acid, a Novel Plant Growth Regulator Isolated from the Mushroom Amanita Castanopsidis Hongo” Y. Morimoto, M. Takaishi, T. Kinoshita, K. Sakaguchi and K. Shibata Chem. Commun. 42-43 (2002).
"Synthesis and Absolute Configuration of Lactone II Isolated from Streptomyces sp. Go 40/10" T. Ueki, Y. Morimoto and T. Kinoshita Chem. Commun. 1820-1821 (2001).
"Total Synthesis and Determination of the Absolute Configuration of (−)-Iongilene Peroxide" Y. Morimoto, T. Iwai and T. KinoshitaTetrahedron Lett. 42, 6307-6309 (2001).
"Stereocontrolled Total Synthesis of the Stemona Alkaloid (−)-Stenine" Y. Morimoto, M. Iwahashi, T. Kinoshita and K. Nishida Chem. Eur. J. 7, 4107–4116 (2001).
“Total Synthesis of (+)-Eurylene and (+)-14-Deacetyl Eurylene” Y. Morimoto, K. Muragaki, T. Iwai, Y. Morishita and T. Kinoshita Angew. Chem., Int. Ed. 39, 4082-4084 (2000).
“Revised Structure of Squalene-Derived Penta THF Polyether, Glabrescol, through Its Enantioselective Total Synthesis: Biogenetically Intriguing CS vs C2 Symmetric Relationships” Y. Morimoto, T. Iwai and T. Kinoshita J. Am. Chem. Soc. 122, 7124-7125 (2000).
"Can α-Sultone Exist as a Chemical Species ? First Experimental Implication for Intermediacy of α-Sultone” Y. Morimoto, H. Kurihara and T. Kinoshita Chem. Commun. 189-190 (2000).
“Effective Combination of Two-Directional Synthesis and Rhenium(VII) Chemistry: Total Synthesis of meso Polyether Teurilene” Y. Morimoto, T. Iwai and T. Kinoshita J. Am. Chem. Soc. 121, 6792-6797 (1999).
“Total Syntheses of Macrocyclic Marine Alkaloids, Haliclamines A and B: A Convenient and Expeditious Assembly of 3-Substituted Pyridine Derivatives with Different Alkyl Chains to the Bispyridinium Macrocycle” Y. Morimoto, C. Yokoe, H. Kurihara and T. Kinoshita Tetrahedron 54, 12197-12214 (1998).
“Highly Diastereoselective Cyclizations of Bishomoallylic Tertiary Alcohols Promoted by Rhenium(VII) Oxide. Critical Steric versus Chelation Effects in Alkoxyrhenium Intermediates” Y. Morimoto and T. Iwai J. Am. Chem. Soc. 120, 1633-1634 (1998).
"Synthesis and Asolute Configuration of (+)-Hyperolactone B" T. Ueki, D. Ichinari, K. Yoshihara, Y. Morimoto and T. Kinoshita Tetrahedron Lett. 39, 667-668 (1998).
"Diastereoselective Two-directional Synthesis and Cation Transport Ability of the Central Tristetrahydrofuranyl Unit of Meso Polyether Glabrescol as Naturally Occurring Podand" Y. Morimoto, T. Iwai, T. Yoshimura, T. Kinoshita Bioorg. Med. Chem. Lett. 8, 2005-2010 (1998).
“Unexpected Stability of δ-Lactones with Axial Substituents rather than Equatorial Ones. Conformational Evaluation by Molecular Mechanics and Molecular Orbital Calculations” Y. Morimoto and H. Shirahama Tetrahedron 53, 2013-2024 (1997).
"Stereocontrolled Synthesis of C10-C22 Fragment of the Iimmunosuppressant FK 506. An Occurrence of Complementary Stereoselectivity in the C15 Ketone Reduction" Y. Morimoto, A. Mikami, S. Kuwabe and H. Shirahama Tetrahedron: Asymmetry 7, 3371-3390 (1996).
“Synthetic Studies on Virantmycin. 2. Total Synthesis of Unnatural (+)-Virantmycin and Determination of Its Absolute Stereochemistry” Y. Morimoto and H. Shirahama Tetrahedron 52, 10631-10652 (1996).
"Synthetic Studies on Virantmycin. 1. Total Synthesis of ()-Virantmycin and Determination of Its Relative Stereochemistry" Y. Morimoto, F. Matsuda and H. Shirahama Tetrahedron 52, 10609-10630 (1996).
“Studies on the Asymmetric Synthesis of Stemona Alkaloids: Total Synthesis of (-)-Stenine” Y. Morimoto, M. Iwahashi, K. Nishida, Y. Hayashi and H. Shirahama Angew. Chem., Int. Ed. 35, 904-906 (1996).

