Research
Development of New Catalytic Systems for Atom-Efficient Asymmetric Reactions
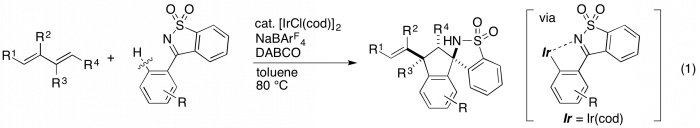
Transition metal-catalyzed functionalization of aromatic compounds via C–H bond activation has realized the step-economy synthesis of useful compounds. In particular, an ortho-selective functionalization has been achieved by use of directing groups, and in the past few decades, many catalytic systems for the C–H bond activation have been developed. We recently reported the iridium-catalyzed [3 + 2] annulation of cyclic ketimines with 1,3-dienes giving spiroaminoindane derivatives, where the reaction proceeds via an aryliridium(I) species generated by the chelation-assisted ortho C−H activation of the aromatic ring (eq 1).1 The use of chiral diene ligands enabled the asymmetric annulation of N-acylketimines with 1,3-dienes.2

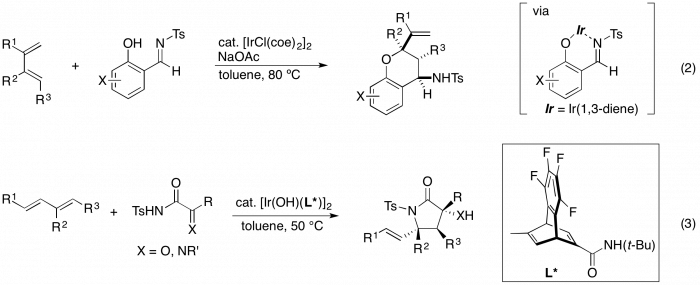
Transition metal-catalyzed intermolecular cycloaddition is one of the most powerful methods for the synthesis of carbo- and heterocyclic compounds. In particular, the development of highly atom-efficient cycloaddition reactions without formation of wastes is a significant objective in synthetic organic chemistry. The annulation reaction of salicylimines with 1,3-dienes in the presence of an Ir catalyst was found to proceed to give 4-aminochromane derivatives with high regio- and stereoselectivity (eq 2).3 An asymmetric annulation with high regio- and enantioselectivity has also been realized by use of an Ir/chiral diene catalyst. We have also developed a highly regio- and enantioselective asymmetric annulation of α-oxocarboxamides and an α-iminocarboxamide with 1,3-dienes catalyzed by hydroxoiridium/chiral diene complexes (eq 3).4

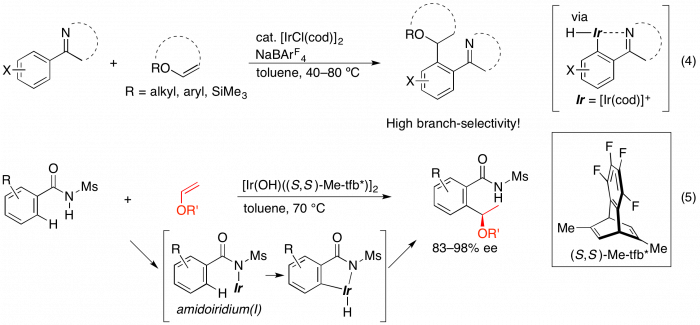
Most of the reports on transition metal-catalyzed hydroarylation reactions via C–H activation have described the addition to alkenes with the linear selectivity. Recently, we reported the iridium-catalyzed annulation of aromatic imines with 1,3-dienes1,2 or alkynes5 via a directed C–H bond activation. In this context, we found that the similar catalytic system is effective in catalyzing a branch-selective hydroarylation of vinyl ethers with aromatic compounds containing nitrogen directing groups (eq 4).6 The use of a chiral diene ligand7 enabled the asymmetric hydroarylation of some vinyl ethers with 2-phenylpyridine to give the corresponding benzylic ethers with good enantioselectivity. We also succeeded in asymmetric alkylation of N-sulfonylbenzamides with vinyl ethers via directed C−H bond activation catalyzed by an Ir/chiral diene complex. The reaction gave high yields of the corresponding addition products with high branch- and enantioselectivity (eq 5).8

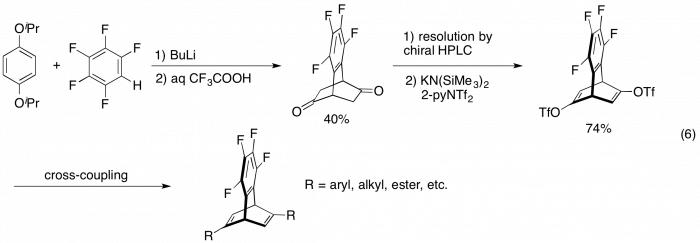
Chiral dienes have been recently developed as a new class of chiral ligands for transition metal catalysts, realizing highly efficient and enantioselective reactions. Of the diene ligands bearing diverse bicyclic skeletons, tetrafluorobenzobicyclo[2.2.2]octatriene (tetrafluorobenzobarrelene; tfb) and its derivatives are attractive compounds because of their high coordination ability toward transition metals. We have developed chiral tfb ligands (eq 6)7 and they have been successfully applied to Rh- and Ir-catalyzed asymmetric reactions.9,10

References
- (a) T. Nishimura, Y. Ebe, T. Hayashi, J. Am. Chem. Soc. 2013, 135, 2092. (b) Y. Ebe, M. Hatano, T. Nishimura, Adv. Synth. Catal. 2015, 357, 1425.
- T. Nishimura, M. Nagamoto, Y. Ebe, T. Hayashi, Chem. Sci. 2013, 4, 4499.
- Y. Ebe, T. Nishimura, J. Am. Chem. Soc. 2014, 136, 9284.
- M. Hatano, T. Nishimura, Angew. Chem., Int. Ed. 2015, 54, 10949.
- M. Nagamoto, T. Nishimura, Chem. Commun. 2014, 50, 6274.
- Y. Ebe, T. Nishimura, J. Am. Chem. Soc. 2015, 137, 5899.
- T. Nishimura, H. Kumamoto, M. Nagaosa, T. Hayashi, Chem. Commun. 2009, 5713.
- M. Hatano, Y. Ebe, T. Nishimura, H. Yorimitsu, J. Am. Chem. Soc. 2016, 38, 4010.
- M. Nagamoto, T. Nishimura, ACS Catal. 2017, 7, 833.
- For recent examples, see: (a) M. Nagamoto, T. Yanagi, T. Nishimura, H. Yorimitsu, Org. Lett. 2016, 18, 4474. (b) T. Nishimura, T. Nagai, R. Takechi, Y. Ebe, Synthesis 2016, 4, 2612. (c) M. Nagamoto, T. Nishimura, Chem. Commun. 2015, 51, 13791. (d) R. Takechi, T. Nishimura, Chem. Commun. 2015, 51, 8528. (e) R. Takechi, T. Nishimura, Org. Biomol. Chem. 2015, 13, 4918.