理学国際教育研究センター(IREC)からのお知らせ
2024年5月10日
- 大学院
- 研究
- 国際交流
- お知らせ
理学国際教育研究センター 研究セミナー情報
下記の通り、理学国際教育研究センター 研究セミナーを開催いたします。
杉本キャンパスと中百舌鳥キャンパスで、それぞれ異なる内容のセミナーを開催いたします。
講師紹介
Jean-Pascal Sutter (LCC-toulouse, The French National Center for Scientific Research (CNRS), France)
専門分野:物質科学
【講演1】
講演タイトル
Open-Frameworks Stitched by Ionic H-Bonds: towards robust and modular porous molecular solids
講演内容
Hydrogen-bonded (H-bonded) porous architectures (also known as HOFs when purely organic) are a rising class of porous molecular materials. The use of H-bonds as supramolecular “cement” generally results in highly crystalline and regenerable solids due to the reversible nature of these bonds.
Our design of supramolecular porous architectures (SPA) follows a bimolecular approach involving protonated amine derivatives (typically poly-pyridines) and anionic metal complexes (ex. metal-oxalate) which respectively act as H-bond donor [D-H]y+ and acceptor [A]x- units, assembled by strong ionic H-bonds. By means of this approach, hybrid frameworks characterized by permanent porosity, large pore size (1 nm, Figure 1), and remarkable thermal and chemical robustness can be obtained [1]. The robust and reliable supramolecular synthons allows altering the substituent (R) of the organic units without compromising the topology of the SPA allowing the formation of series of isostructural porous frameworks with pore-walls decorated by polar or apolar, or mixture of both R groups.[2] Such alterations allow to adjust the sorption characteristics of the SPA but also to implement a specific affinity towards guest molecules.[3] This SPA can also be used as metal-reservoir and matrix for the controlled growth of inorganic nano-crystals.[4]
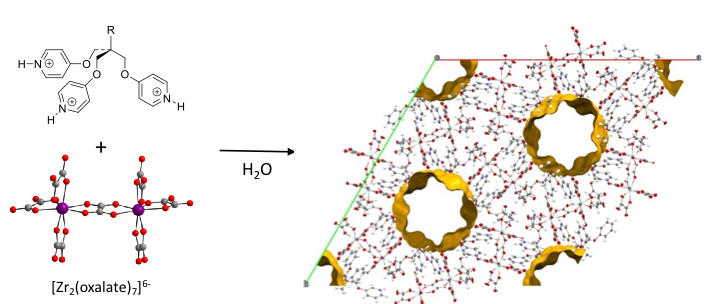
Figure. SPA-1(CH3) framework (R = CH3, H2O located in the channels have been omitted for clarity)
References
[1] Roques, N.; Mouchaham, G.; Duhayon, C.; Brandès, S.; Tachon, A.; Weber, G.; Bellat, J. P.; Sutter, J.-P. Chem. Eur. J. 2014, 20, 11690.
[2] Roques, N.; Tovar-Molle, A.; Duhayon, C.; Brandès, S.; Spieß, A.; Janiak, C.; Sutter, J.-P. Chem. Eur. J. 2022, 61, e202201935.
[3] Mouchaham, G.; Roques, N.; Khodja, W.; Duhayon, C.; Coppel, Y.; Brandès, S.; Fodor, T.; Meyer, M.; Sutter, J.-P. Chem. Eur. J. 2017, 23, 11818.
[4] Khodja, W.; Collière, V.; Kahn, M. L.; Roques, N.; Sutter, J.-P. Chem. Eur. J. 2019, 25, 13705.
場所日時
5/21(火)17:00~18:30
杉本キャンパス、G棟サイエンスホール
【講演2】
講演タイトル
From magnetic anisotropy to molecular magnets: A journey into the coordination chemistry of pentagonal bipyramidal complexes
講演内容
Magnetic anisotropy plays an important role in the behavior of magnetic materials. For instance, the hardness (i.e. coercive field) of bulk magnets is mainly proportional to the magnetic anisotropy, and the energy barrier for magnetization reversal of molecular nanomagnets, such as Single-Molecule Magnets (SMM) and Single-Chain Magnets (SCM), is intimately related to the zero-field splitting characteristics (D) of its individual building units. However, it is still challenging for chemists to control the actual magnetic anisotropy of the complexes, all the more so when they get involved in the construction of heterometallic magnets.
In this context, seven-coordinated 3d ion complexes with pentagonal bipyramidal (PBP) geometry have been found of great relevance. Small or large ZFS D values (up to about +/- 30 cm-1) with a positive or negative sign can be rationally obtained simply by selecting the appropriate metal center.1 After a brief introduction on the origin and control of the magnetic anisotropy in this geometry, we will discuss the interest of such complexes for the construction of heterometallic SMMs and SCMs,2 and some important aspects to be taken into account when designing such systems.3 A recent development towards chiral nanomagnets will also be presented.4
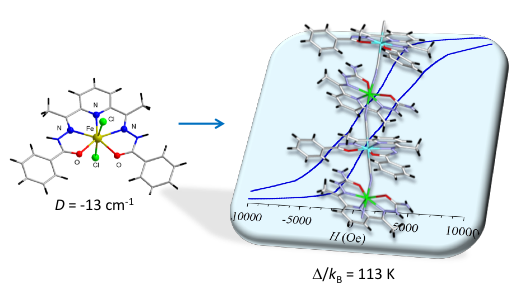
Figure 1.Typical building block of controlled anisotropy and its assemblage with ferromagnetic coupling units to achieve a SCM.
References
1 J.-P. Sutter et al. Chem. Soc. Rev. 2022, 51, 3280.
2 K. Bretosh, et al. Inorg. Chem. Front. 2020, 7, 1503 ; C. Pichon, et al. J. Am. Chem. Soc. 2018, 140, 7698.
3 C. Pichon et al. Chem. Eur. J., 2021, 27, 15484
4 V. Jubault et al. Cryst. Growth&Des. 2023, 23, 1229.
場所日時
5/24(金)17:00~18:30、
中百舌鳥キャンパス サイエンスホール
担当者:細越 裕子