2024
- (44) Copper Complexes with Protein-Based N-Donor Ligands as cis-Selective Nascent Cyclopropanases
Nobutaka Fujieda*, Atsuki Matsuo, Shinobu Itoh*
Chem. Eur. J., xxx, xxxx (2024). in press. 10.1002/chem.202402803.[Link]
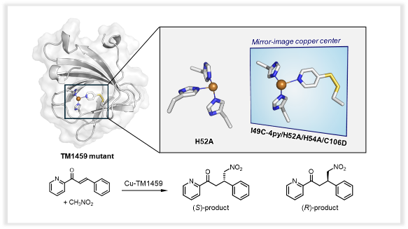
- (43) A thiopyridine-bound mirror-image copper center in an artificial non-heme metalloenzyme
Yoshitsugu Morita*, Hiroki Kubo, Ryusei Matsumoto, Nobutaka Fujieda*
J. Inorg. Biochem., 260, 112694 (2024). 10.1016/j.jinorgbio.2024.112694. [Link]
- (42) Asymmetric Michael addition catalysed by copper–amyloid complexes
Nobutaka Fujieda*, Atsushi Tonomura, Tomofumi Mochizukic, Shinobu Itoh*
RSC Adv., 14 (1), 206–210 (2024). 10.1039/D3RA07900G [Link]
2023
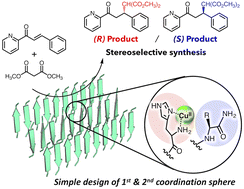
- (41) An Artificial Metallolyase with Pliable 2-His-1-Carboxylate Facial Triad for Stereoselective Michael Addition
Ryusei Matsumoto, Saho Yoshioka, Miho Yuasa, Yoshitsugu Morita, Genji Kurisu, Nobutaka Fujieda*
Chem. Sci., 14 (14), 3932–3937 (2023). 10.1039/D2SC06809E [Link]
2020
- (40) Copper–Oxygen Dynamics in the Tyrosinase Mechanism
Nobutaka Fujieda*, Kyohei Umakoshi, Yuta Ochi, Yosuke Nishikawa, Sachiko Yanagisawa, Minoru Kubo, Genji Kurisu, Shinobu Itoh*
Angew. Chem. Int., 59 (32), 13385–13390 (2020). 10.1002/anie.202004733 [Link]

- (39) Cupin Variants as a Macromolecular Ligand Library for Stereoselective Michael Addition of Nitroalkanes
Nobutaka Fujieda*, Haruna Ichihashi, Miho Yuasa, Yosuke Nishikawa, Genji Kurisu, Shinobu Itoh*
Angew. Chem. Int., 59 (20), 7717–7720 (2020). 10.1002/anie.202000129 [Link]

2017
- (38) A Well-Defined Osmium-Cupin Complex: Hyperstable Artificial Osmium Peroxygenase
Nobutaka Fujieda*, Takumi Nakano, Yuki Taniguchi, Haruna Ichihashi, Hideki Sugimoto, Yuma Morimoto, Yosuke Nishikawa, Genji Kurisu, and Shinobu Itoh*
J. Am. Chem. Soc., 139 (14), 5149–5155 (2017). 10.1021/jacs.7b00675
2016
- (37) Controlling Dicopper Protein Functions
Nobutaka Fujieda and Shinobu Itoh*
Bull. Chem. Soc. Jpn., 89 (7), 733–742 (2016). 10.1246/bcsj.20150444
2015
- (36) Fungal Tyrosinase
Nobutaka Fujieda and Shinobu Itoh*
In Encyclopedia of Inorganic and Bioinorganic Chemistry, R. Huber; Poulos, T.; K.Wieghardt, Eds.; John Wiley & Sons, Ltd: Chichester, UK, 1–8 (2015). 10.1246/bcsj.20150444 - (35) Enzyme Repurposing of a Hydrolase as an Emergent Peroxidase upon Metal Binding
Nobutaka Fujieda*, Jonas Schätti, Edward Stuttfeld, Kei Ohkubo, Timm Maier, and Thomas R. Ward*
Chem. Sci., 6 (34), 4060–4065 (2015). 10.1039/C5SC01065A - (34) Generation, Characterization, and Reactivity of a CuII−Alkylperoxide/Anilino Radical Complex: Insight into the O−O Bond Cleavage Mechanism
Sayantan Paria, Takehiro Ohta, Yuma Morimoto, Takashi Ogura, Hideki Sugimoto, Nobutaka Fujieda, Kei Goto, Kaori Asano, Takeyuki Suzuki, and Shinobu Itoh*
J. Am. Chem. Soc., 137 (34), 10870–10873 (2015). 10.1021/jacs.5b04104 - (33) Direct Hydroxylation of Benzene to Phenol Using Hydrogen Peroxide Catalyzed by Nickel Complexes Supported by Pyridylalkylamine Ligands
Yuma Morimoto, Shuji Bunno, Nobutaka Fujieda, Hideki Sugimoto, and Shinobu Itoh*
J. Am. Chem. Soc., 137 (18), 5867-5870 (2015). 10.1021/jacs.5b01814 - (32) Redox behavior of novel nickel and palladium complexes supported by trianionic non-innocent ligand containing β-diketiminate and phenol groups
Yuma Morimoto, June Takaichi, Shinichi Hanada, Kei Ohkubo, Hideki Sugimoto, Nobutaka Fujieda, Shunichi Fukuzumi and Shinobu Itoh*
J. Porphyrins Phthalocyanines, 19, 377–387 (2015). 10.1142/S1088424615500248
2014
- (31) Geometric Control of Nuclearity in Copper(I)/Dioxygen Chemistry
Tsukasa Abe, Yuma Morimoto, Tetsuro Tano, Kaoru Mieda, Hideki Sugimoto, Nobutaka Fujieda, Takashi Ogura, and Shinobu Itoh*
Inorg. Chem., 53 (16), 8786-8794 (2014). 10.1021/ic501461n - (30) Redox Chemistry of Nickel(II)-Complexes Supported by a Series of Non-innocent β-Diketiminate Ligands
June Takaichi, Yuma Morimoto, Kei Ohkubo, Chizu Shimokawa, Takayuki Hojo, Seiji Mori, * Haruyasu Asahara, Hideki Sugimoto, Nobutaka Fujieda, Nagatoshi Nishiwaki, Shunichi Fukuzumi, and Shinobu Itoh*
Inorg. Chem., 53 (12), 6159-6169 (2014). 10.1021/ic5006693
2013
- (29) Redox Properties of Mononuclear Copper(II)-Superoxide Complex
Tetsuro Tano, Yuri Okubo, Atsushi Kunishita, Minoru Kubo, Hideki Sugimoto, Nobutaka Fujieda, Takashi Ogura, and Shinobu Itoh*
Inorg. Chem., 52 (18), 10431-10437 (2013). 10.1021/ic401261z - (28) Crystal Structures Of Copper-Depleted And Copper-Bound Fungal Pro-Tyrosinase: Insights Into Endogenous Cysteine-Dependent Copper Incorporation
Nobutaka Fujieda, Shintaro Yabuta, Takuya Ikeda, Takuji Oyama, Norifumi Muraki, Genji Kurisu,* and Shinobu Itoh*
J. Biol. Chem., 288 (30), 22128-22140 (2013). 10.1074/jbc.M113.477612 - (27) Copper Complexes of the Non-innocent β-Diketiminate Ligand Containing Phenol Groups
June Takaichi, Kei Ohkubo, Hideki Sugimoto, Motohiro Nakano, Daisuke Usa, Hiroaki Maekawa, Nobutaka Fujieda, Nagatoshi Nishiwaki, Shu Seki, Shunichi Fukuzumi, and Shinobu Itoh*
Dalton Trans., 41 (7), 2438-2444 (2013). 10.1039/C2DT32413J - (26) Activation Mechanism of MelB Tyrosinase from Aspergillus oryzae by Acidic Treatment
Nobutaka Fujieda,* Michiaki Murata, Shintaro Yabuta, Takuya Ikeda, Chizu Shimokawa, Yukihiro Nakamura, Yoji Hata, Shinobu Itoh*
J. Biol. Inorg. Chem., 18 (1), 19-26 (2013). 10.1007/s00775-012-0945-5
2012
- (25) Active Site Models for the CuB Site of Peptidylclycine -Hydroxylating Monooxygenase and Dopamine -Monooxygenase
Atsushi Kunishita, Mehmed Z. Ertem, Yuri Okubo, Tetsuro Tano, Hideki Sugimoto, Kei Ohkubo, Nobutaka Fujieda, Shuichi Fukuzumi, Christopher J. Cramer,* and Shinobu Itoh*
Inorg. Chem., 51 (17), 9465-9480 (2012). 10.1021/ic301272h - (24) Heterolytic Alkylhydroperoxide O–O Bond Cleavage by Copper(I) Complexes
Tetsuro Tano, Hideki Sugimoto, Nobutaka Fujieda, and Shinobu Itoh*
Eur. J. Inorg. Chem., 2012 (26), 4099–4103 (2012). 10.1002/ejic.201200555 - (23) Copper(I)-Dioxygen Reactivity in a Sterically Demanding Tripodal Tetradentate tren Ligand. Formation and Reactivity of a Mononuclear Copper(II) End-on Superoxo Complex
Yuki Kobayashi, Kei Okubo, Takashi Nomura, Minoru Kubo, Nobutaka Fujieda, Hideki Sugimoto, Shunichi Fukuzumi, Kei Goto, Takashi Ogura, and Shinobu Itoh*
Eur. J. Inorg. Chem., 2012 (29), 4574-4578 (2012). [Cover Picture] 10.1002/ejic.201200177 - (22) Artificial Dicopper Oxidase: Rational Reprogramming of Bacterial Metallo-β-lactamase into a Catechol Oxidase
Nobutaka Fujieda,* Atsushi Hasegawa, Kenichi Ishihama, and Shinobu Itoh*
Chem. Asian J., 7 (6), 1203–1207 (2012). [Special Issue of 150 years of German–Japanese friendship] 10.1002/asia.201101014 - (21) Multifunctions of MelB, a Fungal Tyrosinase from Aspergillus oryzae
Nobutaka Fujieda,* Michiaki Murata, Shintaro Yabuta, Takuya Ikeda, Chizu Shimokawa, Yukihiro Nakamura, Yoji Hata, and Shinobu Itoh*
ChemBioChem, 13 (2), 193–201 (2012).
2011
- (20) Reactivity of Copper(II)-alkylperoxo Complexes.
Tetsuro Tano, Mehmed Z. Ertem, Satoru Yamaguchi, Atsushi Kunishita, Hideki Sugimoto, Nobutaka Fujieda, Takashi Ogura, Christopher J. Cramer,* and Shinobu Itoh*
Dalton Trans., 40 (40), 10326–10336 (2011). Front Cover Picture, Hot Article - (19) Post-translational His-Cys Cross Linkage Formation in Tyrosinase Induced by Copper(II)-Peroxo Species.
Nobutaka Fujieda, Takuya Ikeda, Michiaki Murata, Sachiko Yanagisawa, Shigetoshi Aono, Kei Ohkubo, Satoshi Nagao, Takashi Ogura, Shun Hirota, Shunichi Fukuzumi, Yukihiro Nakamura, Yoji Hata, and Shinobu Itoh*
J. Am. Chem. Soc., 133 (5), 1180–1183 (2011). - (18) Reactions of Copper(II)-Phenol Systems with O2. Models for TPQ Biosynthesis in Copper Amine Oxidases.
Kae Tabuchi, Mehmed Z. Ertem, Hideki Sugimoto, Atsushi Kunishita, Tetsuro Tano, Nobutaka Fujieda, Christopher J. Cramer*, and Shinobu Itoh*
Inorg. Chem., 50 (5), 1633–1647 (2011).
2010
- (17) Five Monomeric Hemocyanin Subunits from Portunus trituberculatus: Purification, Spectroscopic Characterization, and Quantitative Evaluation of Phenol Monooxygenase Activity.
Nobutaka Fujieda, Aki Yakiyama, and Shinobu Itoh*
Biochem. Biophys. Acta, 1804, 2128-2135 (2010). - (16) Oxygenation Chemistry at Mononuclear Copper(II)-Hydroquinone Sysetm with O2.
Kae Tabuchi, Hideki Sugimoto, Atsushi Kunishita, Nobutaka Fujieda, and Shinobu Itoh*
Inorg. Chem. 49 (15), 6820–6822 (2010). - (15) Catalytic Oxygenation of Phenols by Arthropod Hemocyanin, an Oxygen Carrier Protein, from Portunus trituberculatus.
Nobutaka Fujieda, Aki Yakiyama, and Shinobu Itoh*
Dalton Trans. 39 (12), 3083–3092 (2010). - (14) Site-directed mutation at residues near the catalytic site of histamine dehydrogenase from Nocardioides simplex and its effects on substrate inhibition.
Maiko Tsutsumi, Noriaki Tsuse, Nobutaka Fujieda, and Kenji Kano*
J. Biochem., 147(2), 257-264 (2010).
2009
- (13) A single circularly permuted GFP sensor for inositol-1,3,4,5-tetrakisphosphate based on a split PH domain.
Reiko Sakaguchi, Takashi Endoh, Seigo Yamamoto, Kazuki Tainaka, Kenji Sugimoto, Nobutaka Fujieda, Shigeki Kiyonaka, Yasuo Mori, and Takashi Morii*
Bioorg. Med. Chem., 17, 7381-7386 (2009).
2008
- (12) The Silent Form of Quinohemoprotein Amine Dehydrogenase from Paracoccus denitrificans.
Nobutaka Fujieda*, Megumi Mori, Tokuji Ikeda, and Kenji Kano
Biosci. Biotech. Biochem., 73(3), 524-529 (2008). - (11) Bioelectrochemical Determination at Histamine Dehydrogenase-based Electrodes.
Yamada, R., Nobutaka Fujieda, Maiko Tsutsumi, Seiya Tsujimura, Osamu Shirai and Kenji Kano*
Electrochemistry, 76(8), 600-602 (2008). - (10) Context-dependent Fluorescence Detection of a Phosphorylated Tyrosine Residue by a Ribonucleopeptide
Tetsuya Hasegawa, Masaki Hagihara, Masatora Fukuda, Shun Nakano, Nobutaka Fujieda and Takashi Morii*
J. Am. Chem. Soc, 130(27), 8804-8812 (2008). - (9) Thermodynamic Redox Properties Governing the Half-reduction Characteristics of Histamine Dehydrogenase from Nocardioides simplex.
Maiko Tsutsumi, Nobutaka Fujieda, Seiya Tsujimura, Osamu Shirai and Kenji Kano*
Biosci. Biotech. Biochem., 72(3), 786-796 (2008).
2007
- (8) Model Studies of 6,7-Indolequinone Cofactors of Quinohemoprotein Amine Dehydrogenases.
Murakami, Y., Yoshimoto, Nobutaka Fujieda, Kei Ohkubo, Kenji Kano, Shunichi Fukuzumi and Shinobu Itoh*
J. Org. Chem., 72(9), 3369-3380 (2007).
2005
- (7) Production of Completely Flavinylated Histamine Dehydrogenase, Unique Covalently Bound Flavin and Iron-Sulfur Cluster Containing Enzyme, of Nocardioides simplex in Escherichia coli and Its Properties.
Nobutaka Fujieda, Noriaki Tsuse, Astuko Satoh, Tokuji Ikeda, and Kenji Kano*
Biosci. Biotech. Biochem., 69(12), 2459-2462 (2005). - (6) Mediated Spectroelectrochemical Titration of Proteins for Redox Potential Measurements by a Separator-less One-compartment Bulk Electrolysis Method.
Seiya Tsujimura, Atsushi Kuriyama, Nobutaka Fujieda, Kenji Kano, and Tokuji Ikeda*
Anal. Biochem., 337 (2), 325-331 (2005).
2004
- (5) 6-S-Cysteinyl Flavin Mononucleotide-containing Histamine Dehydrogenase from Nocardioides simplex: Molecular Cloning, Sequencing, Over-Expression and Characterization of Redox Centers of Enzyme.
Nobutaka Fujieda, Astuko Satoh, Noriaki Tsuse, Kenji Kano, and Tokuji Ikeda*
Biochemistry, 43 (33), 10800-10808 (2004). - (4) Separator-less One-compartment Bulk Electrolysis with a Small Auxiliary Electrode and Its Application to Spectroelectrochemistry.
Atsushi Kuriyama, Moriaki Arasaki, Nobutaka Fujieda, Seiya Tsujimura, Kenji Kano, and Tokuji Ikeda*
Electrochemistry, 72 (7), 484-486 (2004). - (3) DsbB Elicits a Red-shift of Bound Ubiquinone during the Catalysis of DsbA Oxidation.
Kenji Inaba, Yoh-hei Takahashi, Nobutaka Fujieda, Kenji Kano, Hideto Miyoshi, and Koreaki Ito*
J. Biol. Chem., 279 (8), 6761-6768 (2004).
2003
- (2) Redox Properties of Quinohemoprotein Amine Dehydrogenase from Paracoccus denitrificans.
Nobutaka Fujieda, Megumi Mori, Kenji Kano, and Tokuji Ikeda*
Biochim. Biophys. Acta, 1647 (1/2), 289-296 (2003).
2002
- (1) Spectroelectrochemical Evaluation of Redox Potentials of Cysteine Tryptophylquinone and Two Hemes c in Quinohemoprotein Amine Dehydrogenase from Paracoccus denitrificans.
Nobutaka Fujieda, Megumi Mori, Kenji Kano, and Tokuji Ikeda*
Biochemistry, 41 (46), 13736-13743 (2002).